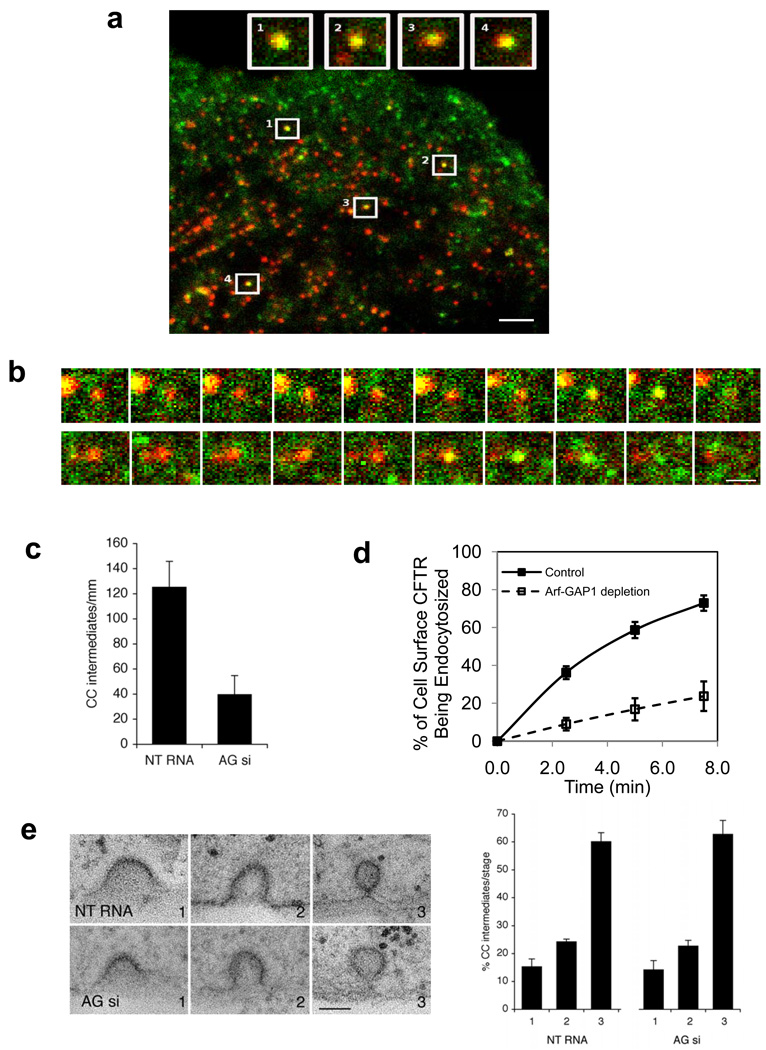
Figure 5. ARFGAP1 affects coated pits formation.
a. Colocalization of ARFGAP1 with clathrin in coated pits. BSC-1 cells transfected with GFP-tagged ARFGAP1 and mCherry-tagged clathrin light chain (CLC) were examined by TIR-FM. The merged view shows colocalization of the two proteins; bar, 2 um. Insets highlight examples of coated pits found to have both ARFGAP1 (green) and clathrin (red).
b. Dynamic association of ARFGAP1 with clathrin in coated pits. BSC-1 cells transfected with GFP-ARFGAP1 and mCherry-CLC and then examined by TIR-FM with live-imaging. Two examples are shown, with images captured every 3 seconds; bar, 1 µm.
c. Depletion of ARFGAP1 reduces the level of coated pits. HeLa cells were treated with siRNA conditions as indicated. All forms of clathrin coated (CC) intermediates were counted and then divided by the length of the plasma membrane. Seven cells were randomly selected from each condition to obtain the mean with standard deviation. Difference between two conditions is significant (p<0.05).
d. Depletion of ARFGAP1 inhibits the endocytosis of CFTR. CFBE41o- cells expressing WT-CFTR were polarized and then treated with siRNA conditions as indicated. The level of internalized CFTR was then quantified. The mean with standard error from three experiments is shown. Difference between two conditions (except time = 0) is significant (p<0.05).
e. Depletion of ARFGAP1 does not induce a particular stage of coated pit formation to accumulate. The different stages of coated pit formation, with representative images for each stage shown (left; bar, 100 nm), were detected by EM, and then quantified. The mean from three experiments with standard deviation is shown (right). Difference between corresponding stages is insignificant (p>0.05).