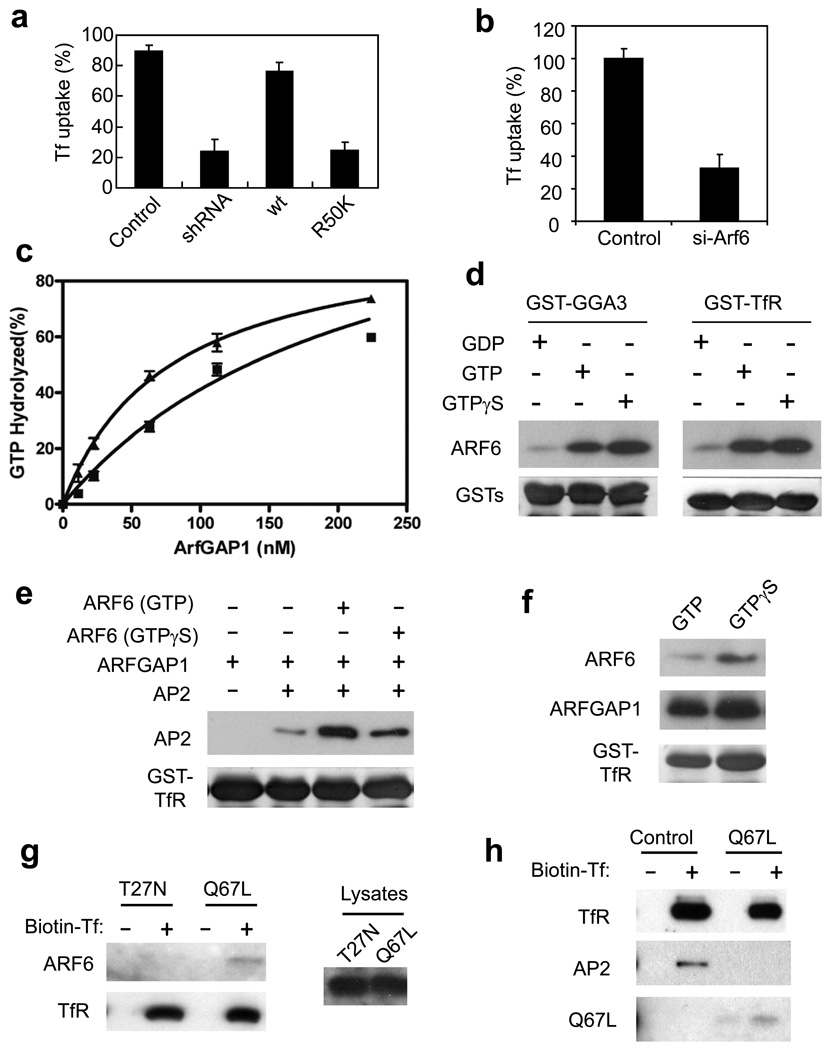
Figure 8. The GAP activity of ARFGAP1 is important for TfR endocytosis.
a. The GAP activity of ARFGAP1 is important for TfR endocytosis. BSC-1 cells that stably expressed shRNA against ARFGAP1 were transfected with constructs as indicated. Uptake of biotin-Tf at 10 minutes was then quantified. The mean with standard error from three experiments is shown. Difference between the condition of shRNA and rescue by Wt is significant (p<0.05). Difference between the condition of shRNA and rescue by R50K is insignificant (p>0.05).
b. Depletion of ARF6 inhibits Tf uptake. BSC-1 cells were treated with siRNA against ARF6. Uptake of biotin-Tf at 10 minutes was then quantified. The mean with standard error from three experiments is shown. Difference between the two conditions is significant (p<0.05).
c. AP-2 enhances GAP activity of ARFGAP1 toward ARF6. The GAP assay was performed using ARF6 as the substrate, and either with (triangles) or without (squares) AP-2. The mean from three experiments with standard error is shown.
d. Binding of ARF6 to TfR is activation-dependent. ARF6 was confirmed functionally for its activation using the effector domain of GGA3 in a pulldown experiment (left panel). ARF6 forms were also incubated with GST-TfR in another pulldown experiment (right panel).
e. GAP activity of ARFGAP1 optimizes the binding of AP-2 to TfR. GST-TfR was incubated sequentially with ARF6 (containing different nucleotide bound, as indicated), followed by ARFGAP1, and then AP-2.
f. Deactivation of ARF6 by ARFGAP1 releases ARF6 from binding to TfR. ARF6 loaded with GTP forms as indicated were incubated with GST-TfR along with ARFGAP1 in a pulldown experiment.
g. Activation-dependent binding of ARF6 to surface TfR. BSC-1 cells were transfected with point mutant forms of ARF6 as indicated. Surface TfR was then isolated through biotin-Tf binding to cell surface, followed by incubation with streptavidin beads. Immunoblotting was then performed for proteins as indicated.
h. Constitutively activated form of ARF6 (Q67L) reduces the binding of AP-2 to surface TfR. BSC-1 cells were transfected with ARF6-Q67L or mock transfected. Surface TfR was then isolated as described above, followed by immunoblotting for associated proteins as indicated.