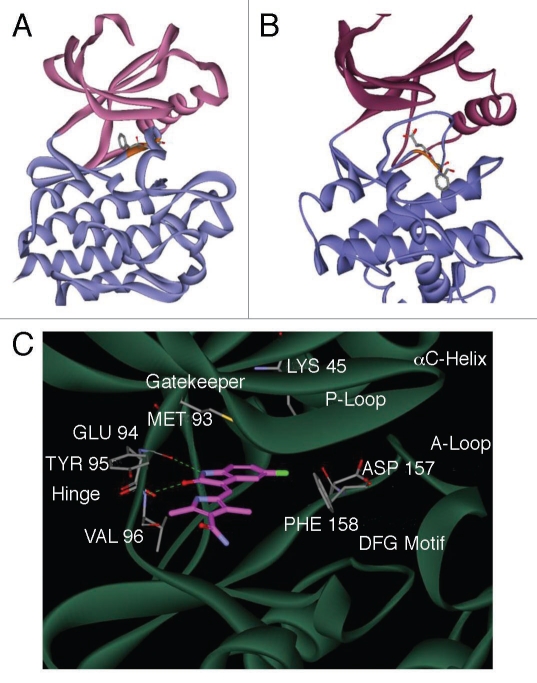
Figure 4.
An energy-minimized computational model of SU11248 bound to the kinase domain of human AMPKα2. Ribbon diagram models of the inactive DFG-out (A) and active DFG-in (B) conformations of the catalytic domain of AMPKα2, showing the N-terminal (pink) and C-terminal (blue) lobes of the subunit. The conserved DFG loop is depicted (orange) with the side chains for the DFG aspartic acid and phenylalanine residues represented as sticks. A detailed view of the active site of AMPKα2:SU11248 (C), showing key hydrogen bonds between SU11248 (magenta carbons) and protein residues (gray carbons) of the ATP-binding hinge region (dashed green lines).