Figure 1. Phosphorylation of Pin1 on Ser71 inhibits its PPIase activity and cellular function.
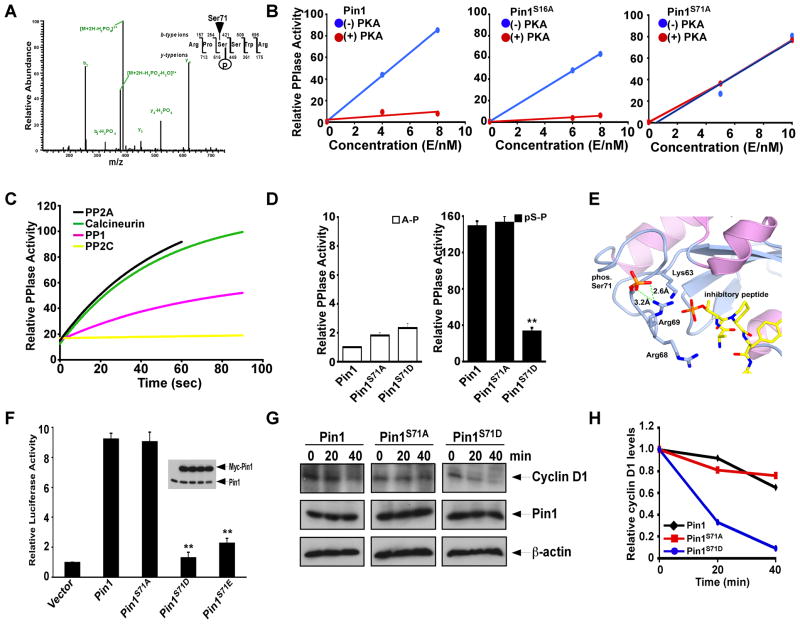
(A) Identification of Ser71 phosphorylation in Pin1 immunoprecipitated from normal breast cells.
(B) Pin1 Ser71 phosphorylation inhibits its PPIase activity. Pin1 and its mutants were phosphorylated by PKA catalytic subunit and then determined their PPIase activities.
(C) Pin1 pSer71 dephosphorylation fully restores its PPIase activity. Dephosphorylation of phosphorylated Pin1 was performed with the Ser/Thr phosphatases PP1, PP2A, PP2B or PP2C.
(D) Pin1-S71D, but not Pin1-S71A mutation abolishes the pSer-Pro specific PPIase activity, without affecting basal Ala-Pro PPIase activity. Results shown are mean ± SEM, n = 3. **, p <0.002.
(E) Structure model of Ser71 inhibitory phosphorylation, with a full view in Figure S1B.
(F) Pin1-S71D and Pin1-S71E mutations abolish the ability to activate cyclin D1 promoter in cells. HeLa cells were co-transfected with Pin1, its mutants, reporter constructs or vector control, followed by assaying the luciferase activity. Results shown are mean ± SEM, n = 3. **, p <0.001.
(G and H) Pin1-S71D mutation abolishes the ability to stabilize endogenous cyclin D1 protein in cells. Pin1−/− MEFs expressing Pin1 or its S71 mutants were treated with cycloheximide, followed by immunoblot with anti-cyclin D1, Pin1 or β-actin antibodies (G). Cyclin D1 levels were semi- quantitated using β-actin as a loading control and the relative levels at time 0 defined as 1 (H).