Figure 1.
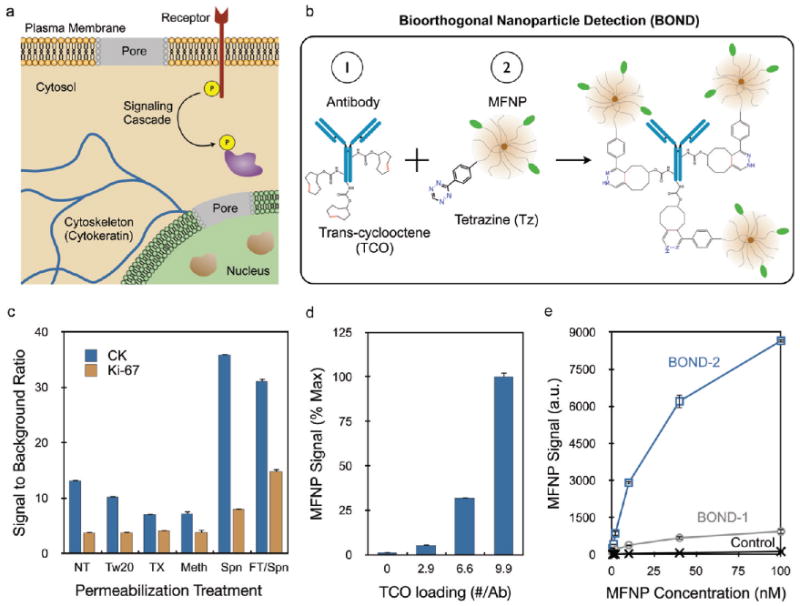
Targeting nanoparticles to intracellular markers using bioorthogonal chemistry coupling. (a) Semipermeabilization of intact cells allows nanoparticle targeting to a variety of intracellular biomarkers as wells as indicators of cell growth, activation, and survival. (b) BOND targeting scheme using a TCO-modified antibody followed by Tz nanoparticle to amplify nanoparticle binding. (c) Investigation of various cell treatments to optimize secondary permeabilization (NT, no treatment; Tw20, Tween 20; TX, Triton X-100; Meth, methanol; Spn, saponin) following fixation. A freeze–thaw treatment prior to fixation was also tested along with saponin (FT/Spn). Optimal MFNP signal-to-background ratios were obtained with the free-ze–thaw/Spn treatment for both cytoplasmic (CK) and nuclear (Ki-67) targets. (d) Increasing the TCO valency on the anti-CK antibody increased MFNP signal. (e) Binding isotherms obtained for targeting CK using BOND-2 and a direct MFNP immuno-conjugate prepared using the TCO/Tz chemistry (BOND-1). Control signals were obtained using Tz-MFNP only. BOND-2 yielded higher MFNP signals at all concentrations, with differences exceeding 10-fold. Error bars represent the standard error from at least three independent experiments.
