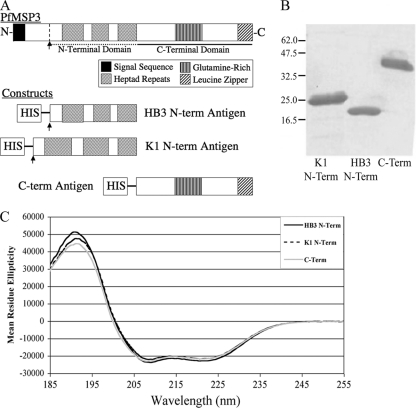
Fig. 1.
Purity and folding of PfMSP3 domain antigens. (A) Three PfMSP3 constructs were generated to test antibody responses by ELISA: HB3 and K1 allele N-terminal domains and the conserved C-terminal domain. The N-terminal domains began at the proteolytic cleavage site and included all three heptad repeats. The C-terminal domain began just C terminal to the last heptad repeat. (B) Coomassie blue-stained SDS-PAGE demonstrates PfMSP3 construct antigens are >95% pure as measured by scanning densitometry. (C) Circular dichroism spectroscopy reveals signature peaks at 215 nm and 190 nm indicative of constructs being comprised largely of alpha-helical structures, consistent with published data for full-length PfMSP3.