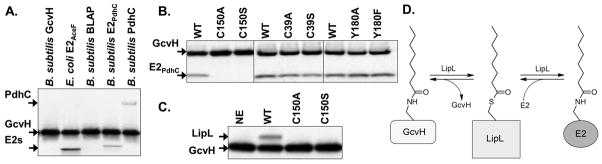
Fig. 4.
Autoradiograms of SDS-PAGE gels of assays for LipL-catalyzed amidotransfer from purified [1-14C]octanoyl-GcvH to lipoyl domains. Panel A: Amidotransfer of the [1-14C]octanoyl moiety from purified octanoyl-GcvH to the unmodified lipoyl domain indicated. Each reaction contained purified wild type (WT) LipL. The two E2 domains migrate similarly and are denoted by E2s. Panel B: Amidotransfer from purified [1-14C]octanoyl-GcvH to the E2PdhC. The purified wild type LipL or point mutant proteins indicated were used as enzyme sources. Panel C: Additional enzyme was added to allow detection of the octanoyl-LipL intermediate. Wild type (WT), C150A point mutant, C150S point mutant LipL were assayed in addition to a control (NE) lacking LipL. Panel D: Schematic of the LipL amidotransfer reaction is shown with the acyl-LipL intermediate.