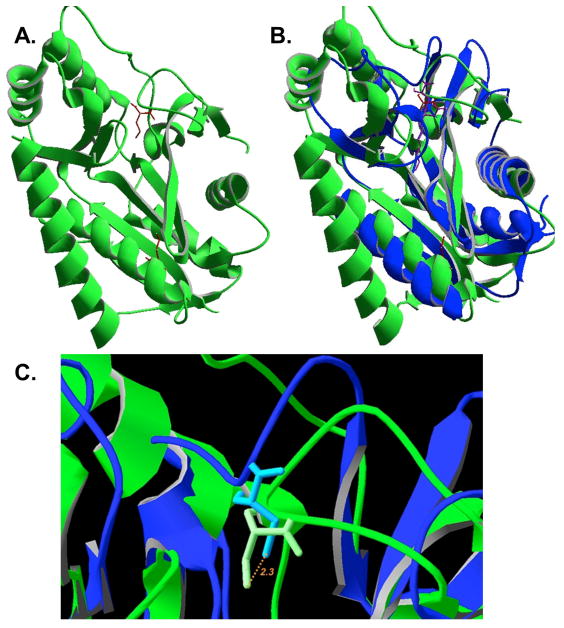
Fig. 9.
Structure of LipL and comparison with that of LipB. Panel A: The unpublished structure of B. halodurans LipL (PDB 2P5I). The modified cysteine residues, C39 and C150, detected by LC-MS/MS of the B. subtilis protein are shown in red. Panel B: Structural alignment of LipL from B. halodurans with M. tuberculosis LipB (PDB 1W66) (Ma et al., 2006). The active site adduct is shown in purple. Panel C: Close up view of the structural overlay with the LipB decanoyl adduct removed. The active site cysteine sulfur atoms are colored orange whereas carbon atoms are white. The distance between the two sulfur atoms is 2.4 Å.