Abstract
The pathways of programmed cell death (PCD) are now understood in extraordinary detail at the molecular level. Although much evidence suggests that they are likely to play a role in Parkinson’s disease (PD), the precise nature of that role remains unknown. Two pathways of cell death that are especially well characterized are cyclin-dependent kinase 5-mediated phosphorylation of myocyte enhancer factor 2 and the mitogen-activated protein kinase signalling cascade. Although blockade of these pathways in animals has achieved a truly remarkable degree of neuroprotection of the neuron cell soma, it has not achieved protection of axons. Thus, there is a need to explore beyond the canonical pathways of PCD and investigate mechanisms of axon destruction. We also need to move beyond the narrow classic concept that the mechanisms of PCD are activated exclusively ‘downstream’, following cellular injury. Studies in the genetics of PD suggest that in some forms of the disease, activation may be an early ‘upstream’ event. Additionally, recent observations suggest that cell death in some contexts may not be initiated by injury, but instead by a failure of intrinsic cell survival signalling. These new points of view offer new opportunities for molecular targeting.
Keywords: Apoptosis, Programmed cell death, LRRK2, DJ-1, Akt, PTEN, GDNF
The concept expressed in the title, that there are cell-autonomous molecular pathways intrinsic to neurons that, if activated, will lead to their destruction, is a relatively recent one in the molecular era of Parkinson’s disease (PD) research. This era can perhaps best be said to have begun just over 50 years ago when Carlsson and his colleagues (1957) first reported the ability of DOPA to reverse the akinetic effect of reserpine. The birth of the concept of programmed cell death (PCD) as a cell-autonomous, ordered molecular pathway of death is more difficult to place in time, because early descriptions of developmental cell death appeared many decades ago (Glucksmann, 1951). However, the molecular basis of these death events was not known until the landmark studies of H. Robert Horvitz and his colleagues in the late 1980s and early 1990s (Ellis et al., 1991). There soon followed an explosion of research on the molecular pathways of PCD (Johnson and Deckwerth, 1993; Raff et al., 1993; Thompson, 1995), and although debate may continue today about the precise role of PCD in PD, there appears to be an emerging consensus over the past 5–10 years that it is quite likely to be an important component of the degenerative process (reviewed in Burke (2008).
Although the concept of PCD is a relatively recent one, there is nevertheless some basis for concern that new and future generations of neuroscientists may take the concept for granted and not realize what a paradigm shift it created in thinking about therapeutic approaches to neuroprotection. Before the concept of PCD was accepted, it was deemed essential to identify the primary cause of PD, whether it might be an environmental toxin or other agent, before interventional steps could be envisioned. However, with the emergence of the concept of PCD, it was realized that even in the absence of knowledge of primary causes (as remains the case today for sporadic PD), effective therapeutic intervention, based on blockade of PCD, may be possible.
Since the groundbreaking work of Horvitz and colleagues, the field of cell death research has amassed an enormous amount of highly detailed and complex information about the many, diverse pathways of PCD. One of the purposes of this review is to take stock of the progress that has been made and to evaluate our prospects for fulfilling the original promise of the PCD concept, to achieve disease-altering therapies for PD. Clearly, we have not yet achieved this promise, and one of our goals is to ask what impediments remain, and what we have learned that will guide our future efforts.
Another goal of this review is to assess how ideas about the relationship between PCD and the degenerative process of PD have evolved over recent years. The original concept was that the primary, unknown cause, or causes, of PD would induce cellular dysfunction that at some point would trigger the mechanisms of PCD, which would then operate ‘downstream’ to bring about the demise of the neuron. While this simple ‘sequential’ model may yet hold true, ideas about the relationships between early neuron dysfunction and PCD in PD have evolved to suggest the possibility of a more ‘upstream’ role. This possibility is suggested by a number of new genetic discoveries in PD that indicate that disease-causing mutations may act by disrupting normal cellular regulation of PCD. Another new point of view is that rather than death pathways being activated secondary to some form of cellular injury, they instead may be the cellular ‘default mode’ and become activated due to the failure of normal suppression by survival signalling. Such a possibility is suggested by some of the gene discoveries, by a number of recent observations on the mechanisms of action of some neurotoxins and by some of the effects described following neurotrophic deprivation in mature mice.
PCD in dopamine neurons: major pathways of neuron destruction
As we have stated, enormous growth in our knowledge of pathways of PCD has shown that they are numerous, diverse and complexly interrelated (Burke, 2008). Adding to this complexity at the molecular level is that the utilization of these diverse paths can change depending on the cellular context in the same cell. For example, within post-mitotic dopamine neurons of the substantia nigra pars compacta (SNpc), while endo-plasmic reticulum stress-related induction of PCD plays an essential role in neurotoxin-induced PCD, it does not in naturally occurring cell death (Silva et al., 2005b). Given the breadth and complexity of the topic, this overview will necessarily be selective, and we have chosen to highlight only two key pathways of death that are especially well substantiated by a diverse and extensive array of experimental observations. We will limit this review to cell death mediators that are cell autonomous to dopaminergic neurons. The role of non-cell-autonomous mediators will be taken up by Dr. Etienne Hirsch in this volume.
Cyclin-dependent kinase-5 and myocyte enhancer factor 2 transcription factors
Cyclin-dependent kinase 5 (Cdk5) is a serine/threonine protein kinase that is structurally related to other Cdks known for their role in regulating the cell cycle (Lew and Wang, 1995; Lew et al., 1992; Meyerson et al., 1992). Unlike these other kinases, however, Cdk5, which is predominantly expressed in neurons (Meyerson et al., 1992), is most highly expressed after mitosis is complete (Tsai et al., 1993). In addition, unlike other Cdks, Cdk5 is not dependent on association with cyclins for activation; instead, it is activated by a brain-specific proteins, p35 (Lew et al., 1994; Tsai et al., 1994) and p39 (Tang et al., 1995). It has been suggested that Cdk5 and p35 play a role in neural differentiation because targeted disruption of the genes results in abnormal development (Chae et al., 1997; Ohshima et al., 1996).
In addition to this role in neural development, Cdk5 has been implicated in apoptosis (Ahuja et al., 1997; Zhang et al., 1997). In brain, we observed high levels of Cdk5 expression in apoptotic profiles in naturally occurring cell death in the substantia nigra (SN) (Henchcliffe and Burke, 1997). We subsequently found that both Cdk5 and p35 are generally expressed in neuronal apoptosis in the SN, induced by diverse forms of injury (Neystat et al., 2001). In all of these contexts, Cdk5 protein was identified within the nucleus. Smith and colleagues (2003) provided evidence of a functional role for Cdk5 in mediating death of dopamine neurons in the chronic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model (Smith et al., 2003). The use of the chronic MPTP model in this investigation is notable and important, because clear morphologic evidence of apoptosis is observed (Tatton and Kish, 1997), unlike the widely used acute model, where apoptosis does not occur (Jackson-Lewis et al., 1995). In the chronic model, MPTP induces an increase in the activity of Cdk5, and toxicity is reduced by Cdk5 blockade, either by pharmacologic inhibition or by use of viral vector-mediated expression of a dominant-negative mutant.
Mechanistic insight into the role of Cdk5 in mediating neurotoxin-induced PCD was first provided by Gong and colleagues (2003), who observed that Cdk5 localized to the nucleus phosphorylates and thereby decreases the activity of the pro-survival transcription factor, myocyte enhancer factor 2 (MEF2). MEF2 was first identified as a regulator of muscle gene expression (Potthoff and Olson, 2007), but subsequent work identified more widespread cellular expression and roles in diverse cellular functions, including proliferation, morphogenesis, differentiation and cell survival. MEF2 proteins (four isoforms have been identified) are expressed in brain, and one of the first activities of MEF2 to be identified in neurons was a role as a mediator of survival due to calcium influx induced by neuronal activity (Mao et al., 1999). In a model of glutamate-induced neurotoxicity, Tang and colleagues (2005) observed that injury was associated with diminished protein levels of MEF2, due to caspase cleavage which, in turn, was dependent on MEF2 phosphorylation by Cdk5. Blockade of Cdk5 activity by means of pharmacologic inhibition or by transduction with a dominant-negative Cdk5 mutant afforded neuroprotection from both glutamate and hydrogenperoxide-induced neurotoxicity (Tang et al., 2005).
Closely related observations have been made in the chronic MPTP model. Crocker and colleagues (Crocker et al., 2003) had previously shown that in this model MPTP induces activation of the calcium-dependent proteases, the calpains. In a subsequent investigation, this group determined that activation of the calpains induces cleavage of the Cdk5-interacting protein p35 to its more active, cleaved form, p25 (Smith et al., 2006). The importance of this activation was determined by investigations of p35 null mice, which revealed a marked resistance to MPTP-induced dopamine neuron loss. As previously demonstrated by Tang and colleagues (2005) for glutamate toxicity, Smith and associates (2006) demonstrated that MPTP, by activation of Cdk5, induced the phosphorylation and subsequent inactivation of MEF2. Blockade of MEF2 phosphorylation occurred in p35 null mice, and neuroprotection was afforded by transduction of SN dopamine neurons with a phosphorylation-resistant mutant form of MEF2.
Thus, these investigators have revealed an important serves of molecular events involving calpain and then Cdk5 activation, leading to MEF2 phosphorylation and inactivation by caspase cleavage. It is important to note, however, that although these molecular processes can be successfully blocked, with protection of neuron cell bodies, such blockade does not afford protection of axons. Neither Cdk5 blockade (Smith et al., 2003) nor calpain inhibition (Crocker et al., 2003) provided protection at the striatal axon level. This inability of approaches based on blockade of apoptosis to provide protection at the axon level is a recurring theme, as discussed further below.
The mixed lineage kinase -c-jun N-terminal kinase signalling cascade
The earliest evidence that the mitogen-activated protein kinase (MAPK) cascade plays an important role in PCD in neurons derived from studies in vitro. These studies have previously been reviewed (Silva et al., 2005a), so it will suffice to mention only the critical highlights. Early investigations demonstrated upregulation of c-jun protein (Ham et al., 1995) and mRNA (Estus et al., 1994) in sympathetic neurons following nerve growth factor (NGF) withdrawal. Treatment of these neurons with a dominant-negative form of c-jun protected them from NGF withdrawal-induced cell death, whereas over-expression of wild-type c-jun protein resulted in significant induction of apoptosis even in the presence of NGF (Ham et al., 1995). Similarly, microinjection of neutralizing antibodies for c-jun protein significantly reduced neuronal death following NGF withdrawal (Estus et al., 1994).
The importance of these early findings has been confirmed by numerous observations in the in vivo context. The earliest studies specifically within the SN in models of death induced by 6-hydroxydopamine (6OHDA) (Jenkins et al., 1993) and by axotomy (Leah et al., 1993) noted substantial and sustained increases in c-jun expression, but these changes were interpreted largely in relation to a possible role in regenerative responses. With subsequent increased awareness of apoptosis as a distinct morphology of PCD (Kerr et al., 1995), it became clear that c-jun expression could be correlated at the cellular level with this form of cell death in living animals. This was true in the context of natural cell death and induced death in the central nervous system (Ferrer et al., 1996a; 1996b). Oo et al. (1999) demonstrated in a post-natal model of apoptosis in the SNpc, induced by early target deprivation, that c-jun and c-jun N-terminal kinase (JNK) expression could be correlated at a cellular level with apoptotic morphology. Thus, these morphologic studies of apoptotic cell death suggested a clear correlation with c-jun expression.
The first principal evidence for a functional role for JNK/c-jun signalling in cell death in living animals derived from studies in JNK null animals. Yang and co-workers (1997b) showed that JNK3 null mice are resistant to kainic acid-induced hippocampal neuron apoptosis. While this study demonstrated a clear role for this JNK isoform in mediating cell death, it remained an open question whether c-jun itself was the relevant substrate for this effect, as other substrates exist. To address this issue, Behrens and colleagues (1999) created mice by homologous recombination in which the endogenous c-jun gene was replaced by an altered gene in which the serines at positions 63 and 73 were replaced by non-phosphorylatable alanines. Mice homozygous for this mutant form of c-jun were also resistant to apoptosis. Thus, the phosphorylation of c-jun by JNK appears to be necessary for apoptosis in this model.
A functional role for c-jun in mediating death specifically within dopamine neurons has been supported by many studies. Crocker et al. (2001) have demonstrated in an axotomy model that adenovirus-mediated expression of a c-jun dominant-negative construct prevents the loss of dopamine neurons. A functional role for JNK/c-jun signalling is also supported by the demonstration that gene transfer of the JNK binding domain of JIP-1 (which inhibits JNK activation) protects dopamine neurons from chronic MPTP toxicity (Xia et al., 2001). In view of this evidence that phosphorylation of c-jun plays a role, and given that JNK is the dominant kinase for c-jun (Kyriakis and Avruch, 2001), it would be predicted that JNK isoforms would also play a role in the death of these neurons. Hunot and co-investigators have shown in a model of acute MPTP toxicity that both JNK2 and JNK3 homozygous null animals are resistant; and compound mutant JNK2 and JNK3 homozygous nulls were even more protected (Hunot et al., 2004). These results, however, are difficult to interpret in specific relation to PCD, because, as mentioned earlier, apoptosis does not occur in the acute MPTP model (Jackson-Lewis et al., 1995).
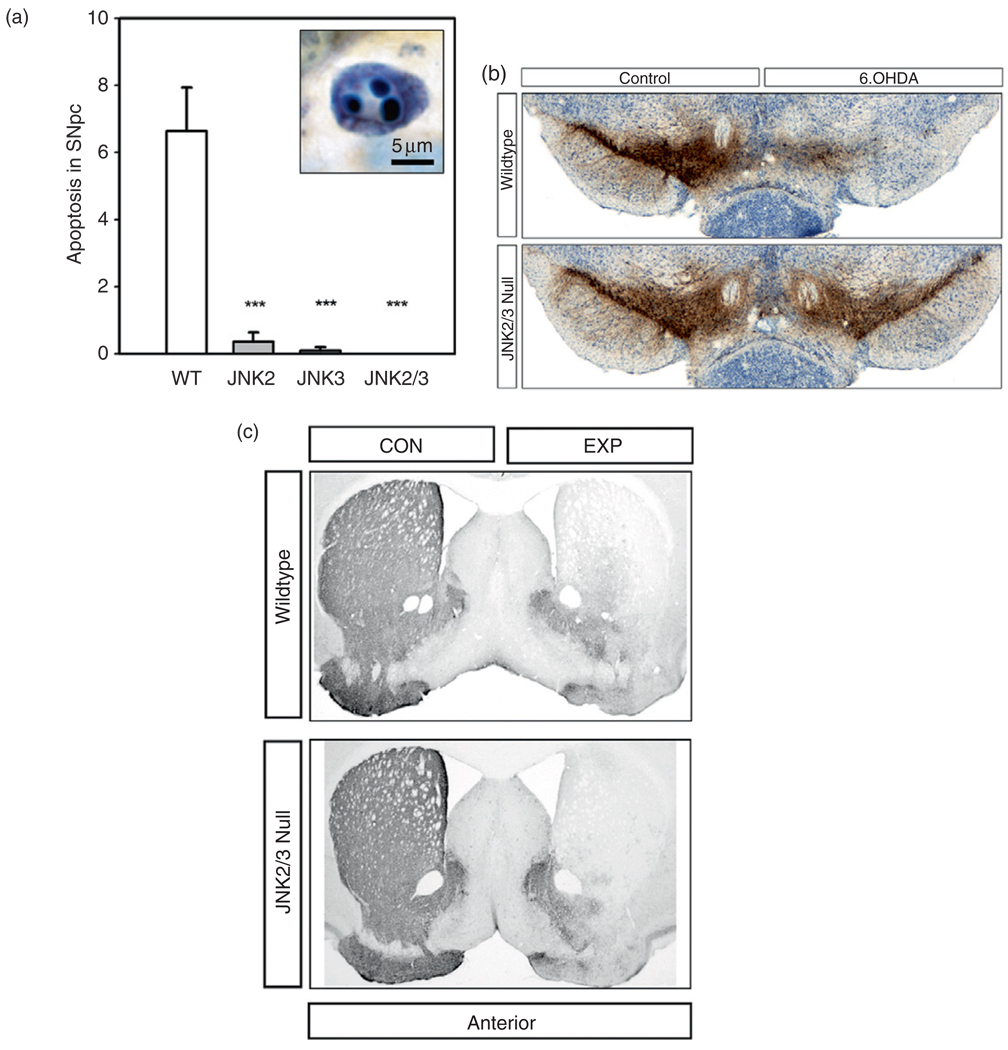
In an alternate model of neurotoxin-mediated degeneration of dopamine neurons, that induced by intra-striatal injection of 6OHDA (Sauer and Oertel, 1994), in which definitive evidence of apoptosis has been observed (Marti et al., 2002), clear protective effects of inhibition of the MAPK signalling cascade have been observed. Chen and colleagues demonstrated that transduction of dopamine neurons of the SN by use of adeno-associated virus (AAV) vectors to express dominant-negative forms of the dual leucine zipper kinases (DLKs) provides a degree of protection of that correlates precisely with the efficiency of transduction and the degree of inhibition of c-jun phosphorylation (Chen et al., 2008). In this investigation, although there was remarkable preservation of dopamine neuron cell bodies, there was no protection of their axon projections. In view of the extensive evidence implicating the MAPK cascade in mediating PCD in dopamine neurons, and yet the important role also of other death signalling pathways, such as the calpain-Cdk5-MEF2 pathway outlined above, the question arises as to whether phosphorylation of c-jun by the JNKs is essential for death to occur. To address this issue, we examined the effect of single or combined JNK2 and JNK3 null mutations on apoptotic death in the 6OHDA model. We observed that while single null mutations substantially inhibit apoptosis in the acute period following toxin injury, neither provides a lasting protection of dopamine neurons; at 4 weeks following injury, there is no detectable neuroprotection (Ries et al., 2008). However, combined homozygous null mutation of JNK2 and JNK3 completely abrogated apoptotic neuron death in this model and provided a virtually complete protection at 4 weeks after lesion. In marked contrast to this striking protection at the level of dopamine neuron cell bodies, there was no protection of their axonal projections (Fig. 1).
Fig. 1.
Resistance of neuron cell bodies, but not axons, to degeneration in JNK null mice. (a) The intra-striatal 6OHDA neurotoxin model induces apoptosis in SN dopamine neurons in wild-type mice. A typical example of an apoptotic profile, with characteristic chromatin clumps, is demonstrated by thionin counterstain in the inset. The homozygous jnk2 and jnk3 single null mutations suppressed apoptosis by 95 and 98%, respectively, and the homozygous jnk2/3 double null mutations completely abrogated apoptosis. (b) The homozygous jnk2/3 double null mutations provided virtually complete protection of SN dopamine neurons. Among wild-type (WT) mice, there was a 63% loss of dopamine neurons, typical for this model, whereas among jnk2/3 nulls, there was only a 4% decrease. Low-power photomicrographs of representative TH-immunostained SN sections from wild-type (top) and jnk2/3 nulls (bottom) following unilateral 6OHDA injection. (c) Homozygous jnk2/3 double null mice are not resistant to retrograde degeneration of nigrostriatal dopaminergic axons induced by intra-striatal 6OHDA. Following 6OHDA, there is a virtually complete loss of TH-positive fibre staining in homozygous jnk2/3 null mice, as in wild-type, throughout the striatum.
Thus, a recurring theme in experimental studies of neuroprotection in animal models of dopaminergic neurodegeneration is that while many approaches based on blockade of PCD have provided robust protection at the cell body level in diverse models, most have fallen short in their ability to protect dopaminergic axonal projections.
Intracellular signalling pathways for dopaminergic axonal degeneration
These observations, that successful protection of cell bodies by blockade of PCD does not also provide protection of axons, should come as no surprise. The concept that important mediators of PCD, such as the caspases, do not play a role in axon degeneration received considerable support from investigations by Finn and colleagues (2000) who noted that caspase-3 is not activated in a variety of models of axon degeneration. These negative results are not universal, because in other contexts, particularly developmental contexts, a role for caspases has been identified (Buki et al., 2000; Cowan et al., 2001; El-Khodor and Burke, 2002; Nikolaev et al., 2009; Srinivasan et al., 1998). Nevertheless, it remains true that experimental measures intended to block apoptosis in adult models of neuron degeneration often prevent cell body but not axonal degeneration, not only in various neurotoxin models of parkinsonism, as we have described (Chen et al., 2008; Eberhardt et al., 2000; Hayley et al., 2004; Ries et al., 2008; Silva et al., 2005b), but also in a genetic model of motor neuron disease (Sagot et al., 1995).
Axon degeneration and PD
This recognition of the distinction between the canonical pathways of PCD and the molecular pathways of axonal destruction, which remain largely unknown, has important implications for neuroprotective therapeutics for PD. We have recently reviewed the evidence to suggest that neurodegeneration in PD begins not in the cell soma, but in the axons (Cheng et al., 2010). Briefly, there is compelling and consistent evidence that at the time of first appearance of motor signs, there is about a 30% loss of total SN neurons in comparison to age-matched controls. On the other hand, multiple lines of evidence suggest that, at first appearance of motor signs, there has been a 50–60% loss of striatal dopaminergic terminals. This assessment is in keeping with observations that, at the time of death, depending on disease duration, while there has been 60–80% loss of SN dopamine neurons (Fearnley and Lees, 1991; Pakkenberg et al., 1991), there has been a much more profound loss of striatal dopaminergic markers (Bernheimer et al., 1973; Kish et al., 1988; Scherman et al., 1989).
The concept that axonal involvement is an early feature of PD is also supported by recent observations made in a bacterial artificial chromosome (BAC) transgenic model based on the expression of hLRRK2(R1441G) (Li et al., 2009). These mice show the development of an age-related hypokinesia by 9–10 months that is reversible by treatment with levodopa. There is no loss of mesencephalic dopamine neurons, but pathology is observed in dopaminergic axons. On tyrosine hydroxylase (TH) immunostaining, the axons are fragmented, they are associated with axonal spheroids, and they form dystrophic neurites (Li et al., 2009). These abnormal axonal features are also observed by staining for abnormally phosphorylated tau. These observations are in keeping with evidence that LRRK2 plays an important role in the regulation of neurite growth and integrity. MacLeod and colleagues (2006) have reported that mutant forms of LRRK2 induce decreases of neurite length in primary neuron culture. Similar observations were made for the LRRK2(G2019S) mutant in neuronally differentiated neuroblastoma cells (Plowey et al., 2008) and in primary neurons derived from transgenic mice (Parisiadou et al., 2009). The molecular basis of these effects is not known, but of potential interest in this regard is the identification of moesin, and the closely allied proteins ezrin and radixin, as possible LRRK2 substrates (Jaleel et al., 2007). These proteins have been implicated in the regulation of neurite outgrowth (Paglini et al., 1998). The ability of LRRK2 to regulate the phosphorylation status of these proteins, and the closely correlated degree of neurite growth, has been observed in primary cultures (Parisiadou et al., 2009).
Thus, based on analysis of the predominant site of pathology in PD at its onset, and evidence from autosomal dominant genetic forms of the disease, it is reasonable to hypothesize that axon dysfunction may be an early feature of PD. Such a possibility may be relevant to understanding why, to date, clinical trials of anti-apoptotic approaches in PD have failed. This point can perhaps be illustrated by consideration of the failure of the PRECEPT neuroprotection trial in PD (The Parkinson Study Group PRECEPT Investigators, 2007). This trial examined the ability of a mixed lineage kinase inhibitor, CEP-1347, to forestall disease progression in early PD. The rationale for the trial was that blockade of the MAPK signalling pathway by a variety of means, including administration of CEP-1347, had been shown to block apoptosis and provide neuroprotection in a variety of PD models (reviewed in Silva et al., 2005a). While there are of course many possible reasons why the trial failed, as previously reviewed (Waldmeier et al., 2006), an important possibility is that although blockade of MAPK signalling blocks apoptosis, it does not protect axons in the mature brain (Chen et al., 2008; Ries et al., 2008). If the brunt of the pathology is at the level of the axons and their terminals throughout the course of the disease, and given also that the terminals are, of course, the principal site of dopamine release, it would follow that it is the progressive degeneration of axons and their terminals, and not neuron loss, that is the primary determinant of clinical progression. If such is the case, then blockade of apoptosis cannot be expected to forestall the clinical progression of the disease.
Inhibition of axon degeneration: is it feasible?
Unlike the remarkably detailed knowledge that we have acquired of the many molecular pathways of PCD, we know exceedingly little of the mechanisms of axon degeneration. In the face of so little information, what hope do we have that we will be able to define these pathways and ultimately provide neuroprotection, just as we have been able to achieve for the cell soma? There is much work to be done, but the feasibility of this goal is suggested by observations in the unique Wallerian degeneration slow (WldS) mutant mouse.
The most striking evidence that axons can survive irrespective of destruction of the neuronal soma derives from observations made in the WldS mouse (Coleman and Perry, 2002). This mutation arose spontaneously in C57Bl/6 mice, and it was demonstrated to cause delayed Wallerian degeneration in peripheral nerve after axotomy (Lunn et al., 1989). The mutation was identified as an 85-kb tandem triplication that results in a novel chimeric mRNA that encodes for the N-terminal 70 amino acids of ubiquitination factor E4B (Ube4B), followed by the complete coding sequence for the nicotinamide adenine dinucleotide (NAD) synthesizing enzyme nicotinamide mononucleotide adenylyltransferase (NMNAT) (Conforti et al., 2000; Mack et al., 2001). It was shown by Deckwerth and Johnson (1994) that axons of sympathetic ganglion neurons derived from WldS mice survive following withdrawal of NGF in spite of induction of apoptosis in the cell soma. The WldS mutation protects axons of many different types of neurons, in diverse species, from a wide variety of injuries, including toxic peripheral neuropathies (Wang et al., 2001) and genetic neuropathies [Mi et al. (2005) and see Coleman (2005) and Luo and O’Leary (2005) for reviews].
These observations suggest that with a deeper understanding of the mechanisms underlying the WldS phenotype, it may be possible to target the molecular pathways of axon degeneration with therapeutic benefit. It is now clear that enzymatic activity of NMNAT is necessary, but not sufficient, for axon protection (Araki et al., 2004; Conforti et al., 2009; MacDonald et al., 2006; Sasaki et al., 2009). In addition to its enzymatic activity, NMNAT appears to require correct cellular targeting. Interestingly, the full protection phenotype can be observed in experiments with NMNAT3, a mitochondrially targeted isoform (Avery et al., 2009; Sasaki et al., 2006; Yahata et al., 2009).
The possibility that the axon protection provided by WldS may be effective within the nigrostriatal dopaminergic system is supported by observations made in neurotoxin models of Parkinsonism. The WldS mutation protects dopaminergic axons, but not cell bodies, from medial forebrain bundle injection of 6OHDA (Sajadi et al., 2004) and injection of MPTP (Hasbani and O’Malley, 2006). We have characterized the WldS phenotype in four models of nigrostriatal axon injury: two that utilize 6OHDA or axotomy to induce anterograde degeneration and two that use these methods to induce retrograde degeneration. Our observations confirm the promise of exploiting WldS -related mechanisms for axon protection, but they also present a new layer of complexity. We find that for both 6OHDA and axotomy, WldS provides protection from anterograde, but not retrograde degeneration (Cheng and Burke, 2010). We observe this protection as preserved immunostaining for TH in axons and striatum, and by structural integrity visualized by green fluorescent protein GFP) in TH-GFP mice. Therefore, while WldS offers the promise of an approach to axon protection, it reveals fundamentally different processes underlying antero- and retrograde degeneration in this system, and the need to explore these mechanisms more deeply.
PCD in dopamine neurons: is it only downstream?
The prevailing concept of the role for PCD in PD has been that its mediators are ‘downstream’ effectors of more proximate and specific causes related to genetic or environmental factors. However, recent studies of four genes that cause inherited forms of Parkinsonism suggest that there may be more intimate and upstream relationships with the mediators of PCD. Mutations in three of these genes, parkin, PINK1, and DJ-1, cause autosomal recessive forms of Parkinsonism, and several lines of evidence suggest that the disease-causing loss-of-function mutations may result in an increased propensity for neurons to die. Mutations in a fourth gene, LRRK2, cause autosomal dominant forms of Parkinsonism, and recent work indicates that they may lead to activation of the extrinsic pathway of PCD. Much of this evidence has recently been reviewed (Burke, 2008), so for our purposes here, we will highlight only two examples of these recent developments.
Mutations in the gene for DJ-1 (PARK7) cause an autosomal recessive early-onset form of familial PD. Bonifati and colleagues first localized the gene for PARK7 in families from Italy and the Netherlands to chromosome 1p36 (Bonifati et al., 2003). They subsequently determined that in the Dutch family a deletion mutation affects the coding region of DJ-1, and in the Italian family a L166P mutation is present and likely to result in loss of function (Bonifati et al., 2003). Human DJ-1 was first identified as an oncogene (Nagakubo et al., 1997), and later determined to be H2O2-responsive, suggesting that it may function as an antioxidant protein (Mitsumoto and Nakagawa, 2001).
Studies in vivo have suggested that one function of DJ-1 may be to negatively regulate apoptotic pathways. In a Drosophila model, Yang and colleagues (2005) have shown that knockdown of the Drosophila DJ-1 homologue in dopaminergic neurons by a transgenic RNAi approach results in a progressive decline in their number and diminished dopamine content. As would be predicted from a number of in vitro studies, DJ-1 knockdown in neurons resulted in increased sensitivity to oxidative stress due to H2O2 exposure. These investigators determined that the neurodegeneration phenotype induced by RNAi knockdown of DJ-1 could be suppressed by co-expression of phosphatidylinositol-3-kinase (PI3K), an upstream mediator of Akt kinase signalling. Conversely, the degenerative phenotype was exacerbated by co-expression of PTEN, a negative regulator of PI3K/Akt signalling. These results are complimented by those of Kim et al. (2005), who demonstrated, also in Drosophila, that DJ-1 serves as a suppresser of PTEN. They also demonstrated in mammalian cells that increased expression of DJ-1 results in increased phosphorylation and activation of Akt, with enhanced cell survival (Kim et al., 2005). Thus, converging lines of evidence suggest that DJ-1 positively regulates the anti-apoptotic Akt kinase pathway.
The mechanism by which DJ-1 may achieve regulation of the Akt signalling pathway is unknown. Clements et al. (2006) have shown that DJ-1 stabilizes Nrf2 protein, a key regulator of antioxidant responses. Protein stabilization is achieved by the ability of DJ-1 to prevent the association of a cytosolic inhibitor, Keap1, with Nrf2, thereby preventing Nrf2 ubiquitination and degradation via the proteasome pathway. The precise mechanism by which DJ-1 prevents Keap1 association with Nrf2, and how, in turn, this effect may be related to activation of the Akt pathway, if at all, is unknown. An alternative possible mechanism by which DJ-1 may regulate PI3K/ Akt signalling is suggested by the observations of van der Brug and colleagues, who identified DJ-1 as an RNA-binding protein (van der Brug et al., 2008). One of the classes of RNAs bound by DJ-1 was members of the PI3K/Akt cascade.
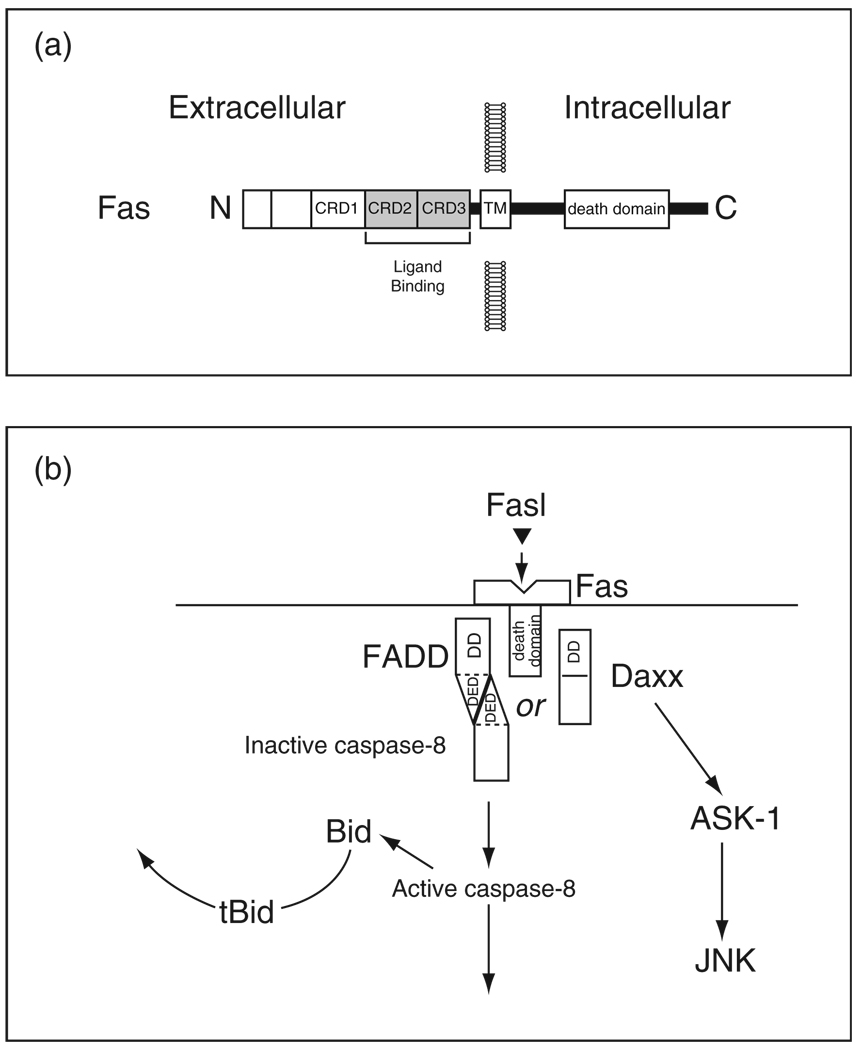
Another example of the close, upstream relationship between a disease-causing gene and the pathways of PCD is the recent demonstration by Ho and colleagues (2009) that LRRK2 interacts with mediators of the extrinsic pathway of PCD. The molecular features of cell death signalling through the extrinsic pathway are less widely studied among neuroscientists than those of the intrinsic pathway, so we will briefly review some of the highlights. PCD is initiated through the extrinsic pathway by the binding of extracellular ligands to receptors (‘death receptors’) which belong to the tumour necrosis factor (TNF) superfamily (Locksley et al., 2001). Members of this family are characterized by a homotrimeric structure and extracellular cysteine-rich domains that interact with ligands that also form homotrimeric assemblies (Fig. 2). For the purposes of this review, we will restrict our attention to a single member of the TNF superfamily: Fas (also known as Apo-1 or CD95). Fas, like other members of the TNF superfamily, is characterized by the presence of intracellular death domains (DDs), which mediate homotypic interactions with other proteins containing DDs, which, in turn, mediate PCD signalling (Fig. 2). Following the binding of the Fas ligand (FasL) to the Fas receptor, signalling is mediated by a variety of pathways (Choi and Benveniste, 2004), but two in particular have been implicated in cell death. In the first, a homotypic interaction occurs between the intracellular DD of Fas and the DD of FADD (Fas-associated protein with DD). FADD contains at its N-terminus a death effector domain (DED) (Tibbetts et al., 2003), which mediates an interaction with a similar DED in pro-caspase-8 (Fig. 2). This interaction permits auto-cleavage, and activation, of caspase-8 by induced proximity. This complex, comprised of ligand, receptor, FADD and procaspase-8 is referred to as the death-inducing signalling complex (DISC) (Wajant, 2003). The cleavage and activation of caspase-8 mediates death by two distinct mechanisms. In some cells, the induced caspase-8 activation is sufficient to mediate cleavage of downstream caspases, such as caspase-3, leading to cell death (Fig. 2). In other cells caspase-8 cleaves a BH3 domain-only member of the Bcl-2 family, Bid. This truncated form (tBid) can induce mitochondrial release of cytochrome c and other mitochondrial mediators of cell death (Fig. 2). An alternate mechanism for mediation of cell death by Fas is initiated by an interaction between the DD of Fas and that of the protein Daxx (Yang et al., 1997a). Unlike FADD, Daxx does not contain a DED. This interaction between Fas and Daxx results in the activation of apoptosis signalling kinase-1, a MAP kinase kinase (Ichijo et al., 1997), which, in turn, activates JNK. Daxx has been suggested to interact with DJ-1 (Junn et al., 2005).
Fig. 2.
Mediation of the extrinsic pathway of PCD by Fas. (a) The protein domain structure of Fas. The receptor has cysteine-rich domains (CRD) in the ligand binding region, characteristic of members of the TNF superfamily of receptors. There is a DD in the intracellular portion of the receptor. (b) Fas death signalling pathways. See text for details.
Ho and colleagues have demonstrated that LRRK2 protein interacts with FADD and that disease-causing mutations enhance this interaction. The functional significance of this interaction was demonstrated by the ability of a dominant-negative form of FADD to block LRRK2-induced cell death. Further exploration of relationships with other members of this death pathway revealed that in the presence of FADD, LRRK2 interacts with caspase-8, and siRNA-based reduction of caspase-8 diminished LRRK2-induced cell death. The disease relevance of these observations was demonstrated by the finding of a cleaved, activated form of caspase-8 in brains of patients with PD due to the LRRK2 mutations.
PCD in dopamine neurons: is it the default mode?
Another aspect of the classic concept of the relationship between neurodegeneration in PD and the processes of PCD that is currently being revised is the notion that initiation of PCD in the disease always requires an initial cellular injury. The hypothesis outlined above for DJ-1, that it normally serves as a positive regulator of PI3K/ Akt signalling, suggests an alternate scenario, that even in the absence of cellular injury, if survival signalling pathways fail in the mature brain, then the processes of PCD become activated. This concept of an ongoing balance within the cell between the processes of cell death and those of cell survival is widely accepted in the context of neuron development. According to classic neurotrophic theory, an immature neuron must achieve target contact and thereby attain neurotrophic support (Oppenheim, 1991). If it fails, survival signalling is not maintained, and it undergoes PCD. However, once target contact is achieved, and the neuron safely survives the developmental cell death period, whether or not it remains dependent on target-derived or alternate sources of trophic support has been unknown. For SN dopamine neurons, developmental target dependence lasts only 2 weeks (Kelly and Burke, 1996). Numerous studies have shown that following extensive striatal target lesion in adulthood, there is no loss of SN dopamine neurons (Krammer, 1980; Lundberg et al., 1994; Stefanis and Burke, 1996).
In spite of this lack of evidence for a need for target-derived support for mature SN dopamine neurons, several recent studies have clearly demonstrated that these neurons continue to depend on neurotrophic support, and, presumably, survival signalling, in the mature brain. Pascual and colleagues (2008) have shown that following conditional deletion of glial cell line-derived neurotrophic factor (GDNF) in mature mice, there is a progressive loss of SN dopamine neurons, such that by 7 months after the deletion, there is a 70% reduction in TH-positive neurons. A similar observation was made by Kramer and colleagues following conditional deletion of the Ret tyrosine kinase in dopamine neurons during development. While the deletion had no effect on the post-natal number of dopamine neurons, it resulted in a 38% loss of dopamine neurons by 2 years of age. This neuron loss was accompanied by loss of striatal dopaminergic innervation and gliosis, particularly in the dorso-lateral quadrant (Kramer et al., 2007). Similar observations have been made for the orphan nuclear receptor Nurr1. Kadkhodaei and colleagues (2009) have shown that conditional deletion of Nurr1 in SN dopamine neurons in mature mice induces a 50% loss of TH-positive neuronal profiles and decreased immunoreactivity in the striatum . In the absence of signs of neuronal degeneration, this loss was attributed to a partial loss of phenotype.
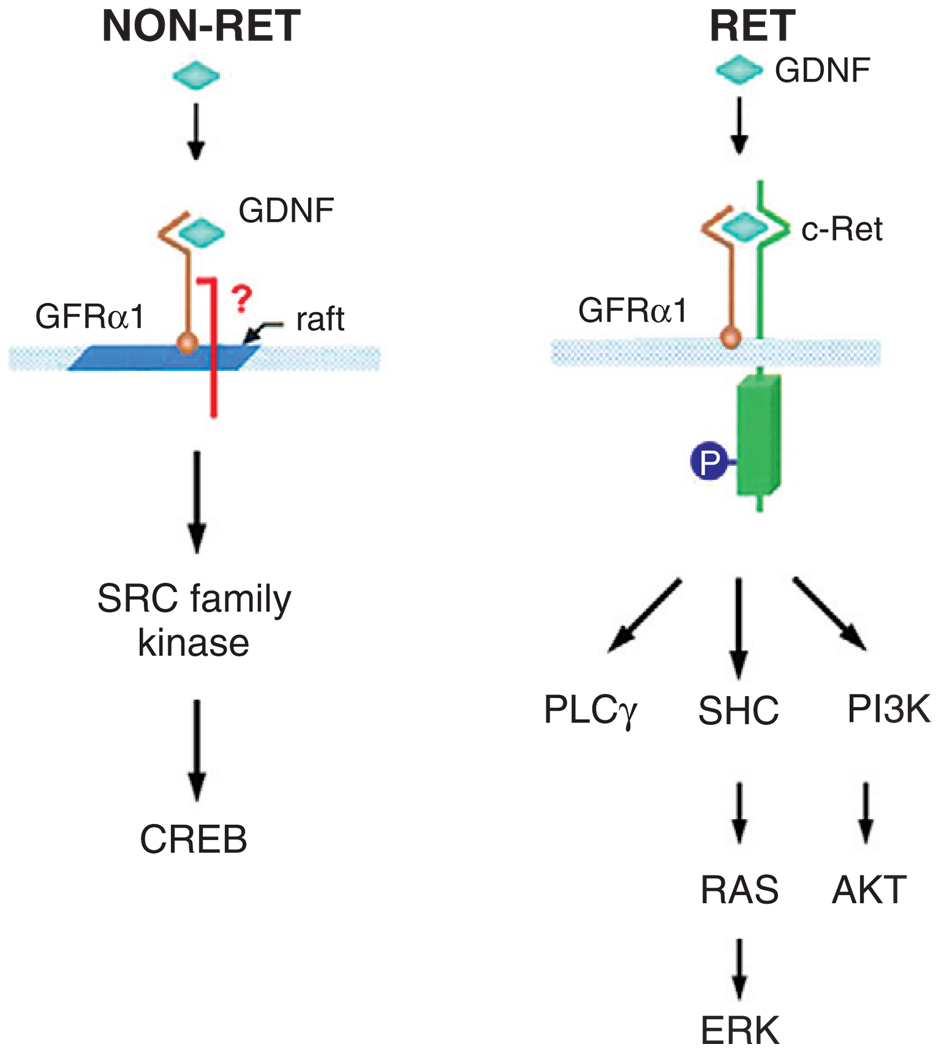
The observations of Pascual et al. (2008) and Kramer et al. (2007) strongly suggest that survival signalling pathways that are activated by GDNF are likely to play a role in maintaining the viability of mature dopamine neurons. The signalling pathways utilized by GDNF to support neuron survival are diverse and complex, so this brief overview will touch only upon the highlights. Although the best characterized pathways are those that are activated following GDNF–GFRxα1 activation of the Ret tyrosine kinase (Fig. 3) [reviewed in Airaksinen and Saarma (2002)], there is considerable evidence that in association with cell membrane lipid rafts, GDNF–GFRα1 may also signal independently of Ret, by activation of Src-family kinases, with resulting activation of cyclic-AMP-response element binding protein (CREB) (Poteryaev et al., 1999; Trupp et al., 1999). Nevertheless, the greater abundance of evidence implicates Ret-dependent signalling, and a variety of downstream pathways, including PLCγ (Trupp et al., 1999) and MEK-ERK1/2 (Cavanaugh et al., 2006; Ugarte et al., 2003), have been implicated in survival signalling (Fig. 3). The most abundant evidence has identified Ret-dependent PI3K/Akt signalling as having a central role in mediating GDNF neuron survival responses (Besset et al., 2000; Chen et al., 2001; Coulpier and Ibanez, 2004; De Vita et al., 2000; Encinas et al., 2001; Perez-Garcia et al., 2004; Pong et al., 1998; Sawada et al., 2000; Soler et al., 1999; Ugarte et al., 2003). A number of these investigations, while finding evidence for a role for PI3K/Akt in mediating survival, failed to find such evidence for ERK1/2 (Besset et al., 2000; Chen et al., 2001; Soler et al., 1999). Especially interesting, in a study of survival signalling mediated at the axon terminal and by retrograde axon transport found that Akt, but not ERK1/2, played a role (Coulpier and Iba-nez, 2004). Nevertheless, PI3K/Akt signalling does not account for all instances of GDNF-mediated survival, and it is clear that cellular context is important. In post-natal mouse sympathetic neurons, GDNF-mediated, Ret-dependent survival requires inhibitor of kappa B kinase rather than PI3K (Encinas et al., 2008).
Fig. 3.
GDNF signalling pathways. In some cellular contexts, GDNF signals independently of RET via interactions in cell membrane rafts with as yet unknown transmembrane proteins, leading to activation of Src-family kinases [see Trupp et al. (1999) and reviewed in Airaksinen and Saarma (2002)]. More often, GDNF signals through GFRα1 interactions with Ret. These interactions result in signalling through several candidate pathways, including PLCγ, Ras-ERKs and PI3K-Akt [modified from Trupp et al. (1999)].
Remarkably little is known about the activity of survival signalling pathways in SN dopamine neurons in vivo. We have shown that a constitutively active form of Akt has marked neurotrophic effects, including an increase in neuron size, expression of phenotypic markers and sprouting, in both mature and aged mice (Ries et al., 2006). In addition, Akt is able to provide marked neuroprotection against 6OHDA-induced apoptosis. However, less is known about the normal physiologic role played by endogenous Akt. During post-natal development, it clearly regulates the magnitude of the developmental cell death event. Transduction of post-natal SN dopamine neurons with a constitutively active form increases the number of these neurons that survive developmental cell death (Ries et al., 2009). Conversely, transduction of these neurons with a dominant-negative form results in an induction of apoptosis and a diminished number of neurons that survive into adulthood. However, whether Akt plays a role in maintenance of survival in the adult setting is unknown.
The possibility that Akt may play such a role is suggested by the recent findings of Greene and colleagues on the mechanism of action of dopaminergic neurotoxins. In a serial analysis of gene expression screen of genes upregulated by 6OHDA in a cellular model, they identified RTP801 (REDD1), a gene implicated in apoptosis in neurons (Malagelada et al., 2006). They determined that RTP801 is induced in the MPTP model and in PD post-mortem SN. The functional significance of its expression in cellular models was demonstrated by an ability of a shRNA approach to provide neuroprotection. Their investigations further showed that RTP801 acts by a general suppression of the mTor kinase, which, in turn, results in dephosphorylation of Akt (Malagelada et al., 2008). The disease relevance of this effect was suggested by their finding that phospho-Akt, but not total Akt, is diminished in PD brain (Malagelada et al., 2008). More recently they have shown that partial and selective suppression of mTor, paradoxically, is protective in both in vitro and in vivo models of neurotoxicity for the reason that it prevents induction of RTP801. This inhibition of RTP801 provides protection by preventing the general inhibition of mTor activity that results in Akt dephosphorylation (Malagelada et al., 2010). The disease relevance of these observations is supported by the recent demonstration that phospho-Akt is normally expressed at high levels in dopamine neurons of the SN, and that it is diminished in PD brain (Timmons et al., 2009). Collectively, these results suggest that an early molecular event in PD may be the loss of survival signalling provided by phosphorylated Akt.
Summary and conclusions
Since the emergence of a molecular understanding of PCD about 20 years ago, its potential to provide a scientific basis for effective neuroprotective therapies for neurodegenerative disorders like PD has remained. The relevance of the concept of PCD to the pathogenesis and treatment of these disorders has not only stood the test of time, it has evolved and generalized. Just as we have been able to define the canonical pathways of PCD, we now can seek to define the pathways of programmed axon degeneration. We now realize that the pathways of PCD may not be confined to a late role of destroying cells after the damage has already been done; they may be intimately involved from the beginning. And finally, we have come to appreciate that even in the adult context, the pathways of PCD are just one side of the survival equation; on the other side, and equally important, are the pathways of cell survival. All of these new views of the concept of PCD offer a multitude of opportunities for the development of new approaches to neuroprotective therapeutics.
Acknowledgements
This work was supported by NS26836, NS38370, the RJG Foundation and the Parkinson’s disease Foundation.
Abbreviations
- 6OHDA
6-hydroxydopamine
- Cdk5
Cyclin-dependent kinase 5
- DD
death domain
- DED
death effector domain
- DISC
death-inducing signaling complex
- DLK
dual leucine zipper kinase
- FADD
Fas-associated protein with DD
- GDNF
glial cell line-derived neurotrophic factor
- JNK
c-jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MEF2
myocyte enhancer factor 2 transcription factors
- NAD
nicotinamide adenine dinucleotide
- NMNAT
nicotinamide mononucleotide adenylyltransferase
- PCD
programmed cell death
- PD
Parkinson’s disease
- PI3K
phosphatidylinositol-3-kinase
- SN
substantia nigra
- SNpc
substantia nigra pars compacta
- TH
tyrosine hydroxylase
- TNF
tumor necrosis factor
- WldS
Wallerian degeneration slow
References
- Ahuja HS, Zhu Y, Zakeri Z. Association of cyclin-dependent kinase 5 and its activator p35 with apoptotic cell death. Developmental Genetics. 1997;21:258–267. doi: 10.1002/(SICI)1520-6408(1997)21:4<258::AID-DVG3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nature Reviews Neuroscience. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- Avery MA, Sheehan AE, Kerr KS, Wang J, Freeman MR. Wld S requires NMNAT1 enzymatic activity and N16-VCP interactions to suppress Wallerian degeneration. The Journal of Cell Biology. 2009;184:501–513. doi: 10.1083/jcb.200808042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens A, Sibilia M, Wagner EF. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nature Genetics. 1999;21:326–329. doi: 10.1038/6854. [DOI] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. Journal of the Neurological Sciences. 1973;20:415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Besset V, Scott RP, Ibanez CF. Signaling complexes and protein-protein interactions involved in the activation of the Ras and phosphatidylinositol 3-kinase pathways by the c-ret receptor tyrosine kinase. The Journal of Biological Chemistry. 2000;275:39159–39166. doi: 10.1074/jbc.M006908200. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Buki A, Okonkwo DO, Wang KK, Povlishock JT. Cytochrome c release and caspase activation in traumatic axonal injury. Journal of Neuroscience. 2000;20:2825–2834. doi: 10.1523/JNEUROSCI.20-08-02825.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE. Programmed cell death and new discoveries in the genetics of parkinsonism. Journal of Neurochemistry. 2008;104:875–890. doi: 10.1111/j.1471-4159.2007.05106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A, Lindqvist M, Magnusson T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature. 1957;180:1200. doi: 10.1038/1801200a0. [DOI] [PubMed] [Google Scholar]
- Cavanaugh JE, Jaumotte JD, Lakoski JM, Zigmond MJ. Neuroprotective role of ERK1/2 and ERK5 in a dopaminergic cell line under basal conditions and in response to oxidative stress. Journal of Neuroscience Research. 2006;84:1367–1375. doi: 10.1002/jnr.21024. [DOI] [PubMed] [Google Scholar]
- Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH. Mice lacking p35, a neuronal specific activator of cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- Chen Z, Chai Y, Cao L, Huang A, Cui R, Lu C, et al. Glial cell line-derived neurotrophic factor promotes survival and induces differentiation through the phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathway respectively in PC12 cells. Neuroscience. 2001;104:593–598. doi: 10.1016/s0306-4522(01)00093-8. [DOI] [PubMed] [Google Scholar]
- Chen X, Rzhetskaya M, Kareva T, Bland R, During MJ, Tank AW, et al. Antiapoptotic and trophic effects of dominant-negative forms of dual leucine zipper kinase in dopamine neurons of the substantia nigra in vivo. Journal of Neuroscience. 2008;28:672–680. doi: 10.1523/JNEUROSCI.2132-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HC, Burke RE. The WldS mutation delays anterograde, but not retrograde, axonal degeneration of the dopaminergic nigro-striatal pathway in vivo. Journal of Neurochemistry. 2010;113:683–691. doi: 10.1111/j.1471-4159.2010.06632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HC, Ulane CM, Burke RE. Clinical progression in Parkinson’s disease and the neurobiology of axons. Annals of Neurology. 2010;67:715–725. doi: 10.1002/ana.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C, Benveniste EN. Fas ligand/Fas system in the brain: regulator of immune and apoptotic responses. Brain Research Reviews. 2004;44:65–81. doi: 10.1016/j.brainresrev.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nature Reviews Neuroscience. 2005;6:889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Perry VH. Axon pathology in neurological disease: a neglected therapeutic target. Trends in Neurosciences. 2002;25:532–537. doi: 10.1016/s0166-2236(02)02255-5. [DOI] [PubMed] [Google Scholar]
- Conforti L, Tarlton A, Mack TG, Mi W, Buckmaster EA, Wagner D, et al. A ufd2/D4Cole1e chimeric protein and overexpression of rbp7 in the slow Wallerian degeneration (WldS) mouse. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11377–11382. doi: 10.1073/pnas.97.21.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L, Wilbrey A, Morreale G, Janeckova L, Beirowski B, Adalbert R, et al. Wld S protein requires NMNAT activity and a short N-terminal sequence to protect axons in mice. The Journal of Cell Biology. 2009;184:491–500. doi: 10.1083/jcb.200807175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulpier M, Ibanez CF. Retrograde propagation of GDNF-mediated signals in sympathetic neurons. Molecular and Cellular Neuroscience. 2004;27:132–139. doi: 10.1016/j.mcn.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Cowan CM, Thai J, Krajewski S, Reed JC, Nicholson DW, Kaufmann SH, et al. Caspases 3 and 9 send a pro-apoptotic signal from synapse to cell body in olfactory receptor neurons. Journal of Neuroscience. 2001;21:7099–7109. doi: 10.1523/JNEUROSCI.21-18-07099.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker SJ, Lamba WR, Smith PD, Callaghan SM, Slack RS, Anisman H, et al. C-Jun mediates axotomy-induced dopamine neuron death in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:13385–13390. doi: 10.1073/pnas.231177098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker SJ, Smith PD, Jackson-Lewis V, Lamba WR, Hayley SP, Grimm E, et al. Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson’s disease. Journal of Neuroscience. 2003;23:4081–4091. doi: 10.1523/JNEUROSCI.23-10-04081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckwerth TL, Johnson EM., Jr Neurites can remain viable after destruction of the neuronal soma by programmed cell death (apoptosis) Developmental Biology. 1994;165:63–72. doi: 10.1006/dbio.1994.1234. [DOI] [PubMed] [Google Scholar]
- De Vita G, Melillo RM, Carlomagno F, Visconti R, Castellone MD, Bellacosa A, et al. Tyrosine 1062 of RET-MEN2A mediates activation of Akt (protein kinase B) and mitogen-activated protein kinase pathways leading to PC12 cell survival. Cancer Research. 2000;60:3727–3731. [PubMed] [Google Scholar]
- Eberhardt O, Coelln RV, Kugler S, Lindenau J, Rathke-Hartlieb S, Gerhardt E, et al. Protection by synergistic effects of adenovirus-mediated X-chromosome-linked inhibitor of apoptosis and glial cell line-derived neurotrophic factor gene transfer in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Journal of Neuroscience. 2000;20:9126–9134. doi: 10.1523/JNEUROSCI.20-24-09126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khodor BF, Burke RE. Medial forebrain bundle axotomy during development induces apoptosis in dopamine neurons of the substantia nigra and activation of caspases in their degenerating axons. The Journal of Comparative Neurology. 2002;452:65–79. doi: 10.1002/cne.10367. [DOI] [PubMed] [Google Scholar]
- Ellis RE, Yuan J, Horvitz HR. Mechanisms and functions of cell death. Annual Review of Cell Biology. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Encinas M, Rozen EJ, Dolcet X, Jain S, Comella JX, Milbrandt J, et al. Analysis of ret knockin mice reveals a critical role for IKKs, but not PI 3-K, in neurotrophic factor-induced survival of sympathetic neurons. Cell Death and Differentiation. 2008;15:1510–1521. doi: 10.1038/cdd.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas M, Tansey MG, Tsui-Pierchala BA, Comella JX, Milbrandt J, Johnson EM., Jr C-src is required for glial cell line-derived neurotrophic factor (GDNF) family ligand-mediated neuronal survival via a phosphatidylinositol-3 kinase (PI-3k)-dependent pathway. Journal of Neuroscience. 2001;21:1464–1472. doi: 10.1523/JNEUROSCI.21-05-01464.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estus S, Zaks WJ, Freeman RS, Gruda M, Bravo R, Johnson EM. Altered gene expression in neurons during programmed cell death identification of c-Jun as necessary for neuronal apoptosis. The Journal of Cell Biology. 1994;127:1717–1727. doi: 10.1083/jcb.127.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Olive M, Blanco R, Cinos C, Planas AM. Selective c-Jun overexpression is associated with ionizing radiation-induced apoptosis in the developing cerebellum of the rat. Molecular Brain Research. 1996a;38:91–100. doi: 10.1016/0169-328x(95)00334-o. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Olive M, Ribera J, Planas AM. Naturally occurring (programmed) and radiation-induced apoptosis are associated with selective c-Jun expression in the developing rat brain. European Journal of Neuroscience. 1996b;8:1286–1298. doi: 10.1111/j.1460-9568.1996.tb01297.x. [DOI] [PubMed] [Google Scholar]
- Finn JT, Weil M, Archer F, Siman R, Srinivasan A, Raff MC. Evidence that Wallerian degeneration and localized axon degeneration induced by local neurotrophin deprivation do not involve caspases. Journal of Neuroscience. 2000;20:1333–1341. doi: 10.1523/JNEUROSCI.20-04-01333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glucksmann A. Cell deaths in normal vertebrate ontogeny. Biology Review. 1951;26:59–86. doi: 10.1111/j.1469-185x.1951.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Gong X, Tang X, Wiedmann M, Wang X, Peng J, Zheng D, et al. Cdk5-mediated inhibition of the protective effects of transcription factor MEF2 in neurotoxicity-induced apoptosis. Neuron. 2003;38:33–46. doi: 10.1016/s0896-6273(03)00191-0. [DOI] [PubMed] [Google Scholar]
- Ham J, Babij C, Whitfield J, Pfarr CM, Lallemand D, Yaniv M, et al. A c-Jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron. 1995;14:927–939. doi: 10.1016/0896-6273(95)90331-3. [DOI] [PubMed] [Google Scholar]
- Hasbani DM, O’Malley KL. Wld(S) mice are protected against the Parkinsonian mimetic MPTP. Experimental Neurology. 2006;202:93–99. doi: 10.1016/j.expneurol.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Hayley S, Crocker SJ, Smith PD, Shree T, Jackson-Lewis V, Przedborski S, et al. Regulation of dopaminergic loss by Fas in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Journal of Neuroscience. 2004;24:2045–2053. doi: 10.1523/JNEUROSCI.4564-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henchcliffe C, Burke RE. Increased expression of cyclin-dependent kinase 5 in induced apoptotic neuron death in rat substantia nigra. Neuroscience Letters. 1997;230:41–44. doi: 10.1016/s0304-3940(97)00472-2. [DOI] [PubMed] [Google Scholar]
- Ho CC, Rideout HJ, Ribe E, Troy CM, Dauer WT. The Parkinson disease protein leucine-rich repeat kinase 2 transduces death signals via fas-associated protein with death domain and caspase-8 in a cellular model of neurodegeneration. Journal of Neuroscience. 2009;29:1011–1016. doi: 10.1523/JNEUROSCI.5175-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunot S, Vila M, Teismann P, Davis RJ, Hirsch EC, Przedborski S, et al. JNK-mediated induction of cyclooxygenase 2 is required for neurodegeneration in a mouse model of Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:665–670. doi: 10.1073/pnas.0307453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6,-tetrahydropyridine. Neurodegen. 1995;4:257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Jaleel M, Nichols RJ, Deak M, Campbell DG, Gillardon F, Knebel A, et al. LRRK2 phosphorylates moesin at threonine-558: characterization of how Parkinson’s disease mutants affect kinase activity. The Biochemical Journal. 2007;405:307–317. doi: 10.1042/BJ20070209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R, O’Shea R, Thomas KL, Hunt SP. C-Jun expression in substantia nigra neurons following striatal 6-hydroxydopamine lesions in the rat. Neuroscience. 1993;53:447–455. doi: 10.1016/0306-4522(93)90208-w. [DOI] [PubMed] [Google Scholar]
- Johnson EM, Deckwerth TL. Molecular mechanisms of developmental neuronal death. Annual Review of Neuroscience. 1993;16:31–46. doi: 10.1146/annurev.ne.16.030193.000335. [DOI] [PubMed] [Google Scholar]
- Junn E, Taniguchi H, Jeong BS, Zhao X, Ichijo H, Mouradian MM. Interaction of DJ-1 with Daxx inhibits apoptosis signal-regulating kinase 1 activity and cell death. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9691–9696. doi: 10.1073/pnas.0409635102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadkhodaei B, Ito T, Joodmardi E, Mattsson B, Rouillard C, Carta M, et al. Nurr1 is required for maintenance of maturing and adult midbrain dopamine neurons. Journal of Neuroscience. 2009;29:15923–15932. doi: 10.1523/JNEUROSCI.3910-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly WJ, Burke RE. Apoptotic neuron death in rat substantia nigra induced by striatal excitotoxic injury is developmentally dependent. Neuroscience Letters. 1996;220:85–88. doi: 10.1016/s0304-3940(96)13216-x. [DOI] [PubMed] [Google Scholar]
- Kerr JFR, Gobe GC, Winterford CM, Harmon BV. Anatomical methods in cell death. In: Schwartz LM, Osborne BA, editors. Methods in cell biology: cell death. New York: Academic Press; 1995. pp. 1–27. [DOI] [PubMed] [Google Scholar]
- Kim RH, Peters M, Jang Y, Shi W, Pintilie M, Fletcher GC, et al. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005;7:263–273. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. The New England Journal of Medicine. 1988;318:876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- Krammer EB. Anterograde and transsynaptic degeneration ‘en cascade’ in basal ganglia induced by intrastriatal injection of kainic acid: an animal analogue of Huntington’s disease. Brain Research. 1980;196:209–221. doi: 10.1016/0006-8993(80)90727-1. [DOI] [PubMed] [Google Scholar]
- Kramer ER, Aron L, Ramakers GM, Seitz S, Zhuang X, Beyer K, et al. Absence of ret signaling in mice causes progressive and late degeneration of the nigrostriatal system. PLoS Biology. 2007;5:e39. doi: 10.1371/journal.pbio.0050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiological Reviews. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Leah JD, Herdegen T, Murashov A, Dragunow M, Bravo R. Expression of immediate early gene proteins following axotomy and inhibition of axonal transport in the rat central nervous system. Neuroscience. 1993;57:53–66. doi: 10.1016/0306-4522(93)90111-r. [DOI] [PubMed] [Google Scholar]
- Lew J, Wang JH. Neuronal cdc2-like kinase. Trends in Biological Science. 1995;20:33–37. doi: 10.1016/s0968-0004(00)88948-3. [DOI] [PubMed] [Google Scholar]
- Lew J, Huang Q-Q, Qi Z, Winkfein RJ, Aebersold R, Hunt T, et al. A brain-specific activator of cyclin-dependent kinase 5. Nature. 1994;371:423–426. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- Lew J, Winkfein RJ, Paudel HK, Wang JH. Brain proline-directed protein kinase is a neurofilament kinase which displays high sequence homology to p34cdc2. The Journal of Biological Chemistry. 1992;267:25922–25926. [PubMed] [Google Scholar]
- Li Y, Liu W, Oo TF, Wang L, Tang Y, Jackson-Lewis V, et al. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson’s disease. Nature Neuroscience. 2009;12:826–828. doi: 10.1038/nn.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Lundberg C, Wictorin K, Bjorklund A. Retrograde degenerative changes in the substantia nigra pars compacta following an excitotoxic lesion of the striatum. Brain Research. 1994;644:205–212. doi: 10.1016/0006-8993(94)91681-0. [DOI] [PubMed] [Google Scholar]
- Lunn ER, Perry VH, Brown MC, Rosen H, Gordon S. Absence of Wallerian degeneration does not hinder regeneration in peripheral nerve. European Journal of Neuroscience. 1989;1:27–33. doi: 10.1111/j.1460-9568.1989.tb00771.x. [DOI] [PubMed] [Google Scholar]
- Luo L, O’Leary DD. Axon retraction and degeneration in development and disease. Annual Review of Neuroscience. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- MacDonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, Freeman MR. The drosophila cell corpse engulfment receptor draper mediates glial clearance of severed axons. Neuron. 2006;50:869–881. doi: 10.1016/j.neuron.2006.04.028. [DOI] [PubMed] [Google Scholar]
- MacLeod D, Dowman J, Hammond R, Leete T, Inoue K, Abeliovich A. The familial parkinsonism gene LRRK2 regulates neurite process morphology. Neuron. 2006;52:587–593. doi: 10.1016/j.neuron.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Mack TG, Reiner M, Beirowski B, Mi W, Emanuelli M, Wagner D, et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/NMNAT chimeric gene. Nature Neuroscience. 2001;4:1199–1206. doi: 10.1038/nn770. [DOI] [PubMed] [Google Scholar]
- Malagelada C, Jin ZH, Greene LA. RTP801 is induced in Parkinson’s disease and mediates neuron death by inhibiting Akt phosphorylation/activation. Journal of Neuroscience. 2008;28:14363–14371. doi: 10.1523/JNEUROSCI.3928-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagelada C, Jin ZH, Jackson-Lewis V, Przedborski S, Greene LA. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson’s disease. Journal of Neuroscience. 2010;30:1166–1175. doi: 10.1523/JNEUROSCI.3944-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagelada C, Ryu EJ, Biswas SC, Jackson-Lewis V, Greene LA. RTP801 is elevated in Parkinson brain substantia nigral neurons and mediates death in cellular models of Parkinson’s disease by a mechanism involving mammalian target of rapamycin inactivation. Journal of Neuroscience. 2006;26:9996–10005. doi: 10.1523/JNEUROSCI.3292-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Bonni A, Xia F, Nadal-Vicens M, Greenberg ME. Neuronal activity-dependent cell survival mediated by transcription factor MEF2. Science. 1999;286:785–790. doi: 10.1126/science.286.5440.785. [DOI] [PubMed] [Google Scholar]
- Marti MJ, Saura J, Burke RE, Jackson-Lewis V, Jimenez A, Bonastre M, et al. Striatal 6-hydroxydopamine induces apoptosis of nigral neurons in the adult rat. Brain Research. 2002;958:185–191. doi: 10.1016/s0006-8993(02)03694-6. [DOI] [PubMed] [Google Scholar]
- Meyerson M, Enders GH, Wu C-L, Su L-K, Gorka C, Nelson C, et al. A family of human cdc2-related protein kinases. The EMBO Journal. 1992;11:2909–2917. doi: 10.1002/j.1460-2075.1992.tb05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi W, Beirowski B, Gillingwater TH, Adalbert R, Wagner D, Grumme D, et al. The slow Wallerian degeneration gene, WldS, inhibits axonal spheroid pathology in gracile axonal dystrophy mice. Brain. 2005;128:405–416. doi: 10.1093/brain/awh368. [DOI] [PubMed] [Google Scholar]
- Mitsumoto A, Nakagawa Y. DJ-1 is an indicator for endogenous reactive oxygen species elicited by endotoxin. Free Radical Research. 2001;35:885–893. doi: 10.1080/10715760100301381. [DOI] [PubMed] [Google Scholar]
- Nagakubo D, Taira T, Kitaura H, Ikeda M, Tamai K, Iguchi-Ariga SM, et al. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochemical and Biophysical Research Communications. 1997;231:509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- Neystat M, Rzhetskaya M, Oo TF, Kholodilov N, Yarygina O, Wilson A, et al. Expression of cyclin-dependent kinase 5 and its activator p35 in models of induced apoptotic death in neurons of the substantia nigra in vivo. Journal of Neurochemistry. 2001;77:1611–1625. doi: 10.1046/j.1471-4159.2001.00376.x. [DOI] [PubMed] [Google Scholar]
- Nikolaev A, McLaughlin T, O’Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ohshima T, Ward JM, Huh C-G, Longenecker G, Veeranna Pant HC, et al. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal cortico-genesis, neuronal pathology and perinatal death. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oo TF, Henchcliffe C, James D, Burke RE. Expression of c-fos, c-Jun, and c-Jun N-terminal kinase (JNK) in a developmental model of induced apoptotic death in neurons of the substantia nigra. Journal of Neuro-chemistry. 1999;72:557–564. doi: 10.1046/j.1471-4159.1999.0720557.x. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW. Cell death during development of the nervous system. Annual Review of Neuroscience. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Paglini G, Kunda P, Quiroga S, Kosik K, Caceres A. Suppression of radixin and moesin alters growth cone morphology, motility, and process formation in primary cultured neurons. The Journal of Cell Biology. 1998;143:443–455. doi: 10.1083/jcb.143.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkenberg B, Moller A, Gundersen HJ, Mouritzen DA, Pakkenberg H. The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson’s disease estimated with an unbiased stereological method. Journal of Neurology, Neurosurgery, and Psychiatry. 1991;54:30–33. doi: 10.1136/jnnp.54.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisiadou L, Xie C, Cho HJ, Lin X, Gu XL, Long CX, et al. Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis. Journal of Neu-roscience. 2009;29:13971–13980. doi: 10.1523/JNEUROSCI.3799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gomez-Diaz R, Lopez-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nature Neuroscience. 2008;11:755–761. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- Perez-Garcia MJ, Cena V, de Pablo Y, Llovera M, Comella JX, Soler RM. Glial cell line-derived neurotrophic factor increases intracellular calcium concentration. Role of calcium/calmodulin in the activation of the phosphatidylinositol 3-kinase pathway. The Journal of Biological Chemistry. 2004;279:6132–6142. doi: 10.1074/jbc.M308367200. [DOI] [PubMed] [Google Scholar]
- Plowey ED, Cherra SJ, III, Liu YJ, Chu CT. Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells. Journal of Neurochemistry. 2008;105:1048–1056. doi: 10.1111/j.1471-4159.2008.05217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pong K, Xu RY, Baron WF, Louis JC, Beck KD. Inhibition of phosphatidylinositol 3-kinase activity blocks cellular differentiation mediated by glial cell line-derived neurotrophic factor in dopaminergic neurons. Journal of Neurochemistry. 1998;71:1912–1919. doi: 10.1046/j.1471-4159.1998.71051912.x. [DOI] [PubMed] [Google Scholar]
- Poteryaev D, Titievsky A, Sun YF, Thomas-Crusells J, Lindahl M, Billaud M, et al. GDNF triggers a novel ret-independent src kinase family-coupled signaling via a GPI-linked GDNF receptor alpha1. FEBS Letters. 1999;463:63–66. doi: 10.1016/s0014-5793(99)01590-2. [DOI] [PubMed] [Google Scholar]
- Potthoff MJ, Olson EN. MEF2: a central regulator of diverse developmental programs. Development. 2007;134:4131–4140. doi: 10.1242/dev.008367. [DOI] [PubMed] [Google Scholar]
- Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD. Programmed cell death and the control of cell survival lessons from the nervous system. Science. 1993;262:695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- Ries V, Cheng HC, Baohan A, Kareva T, Oo TF, Rzhetskaya M, et al. Regulation of the postnatal development of dopamine neurons of the substantia nigra in vivo by akt/protein kinase B. Journal of Neurochemistry. 2009;110:23–33. doi: 10.1111/j.1471-4159.2009.06101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries V, Henchcliffe C, Kareva T, Rzhetskaya M, Bland RJ, During MJ, et al. Oncoprotein akt/PKB: trophic effects in murine models of Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18757–18762. doi: 10.1073/pnas.0606401103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries V, Silva RM, Oo TF, Cheng HC, Rzhetskaya M, Kholodilov N, et al. JNK2 and JNK3 combined are essential for apoptosis in dopamine neurons of the substantia nigra, but are not required for axon degeneration. Journal of Neurochemistry. 2008;107:1578–1588. doi: 10.1111/j.1471-4159.2008.05713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagot Y, Dubois-Dauphin M, Tan SA, de Bilbao F, Aebischer P, Martinou JC, et al. Bcl-2 overexpression prevents motoneuron cell body loss but not axonal degeneration in a mouse model of a neurodegenerative disease. Journal of Neuroscience. 1995;15:7727–7733. doi: 10.1523/JNEUROSCI.15-11-07727.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajadi A, Schneider BL, Aebischer P. Wlds-mediated protection of dopaminergic fibers in an animal model of parkinson disease. Current Biology. 2004;14:326–330. doi: 10.1016/j.cub.2004.01.053. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Araki T, Milbrandt J. Stimulation of nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy. Journal of Neuroscience. 2006;26:8484–8491. doi: 10.1523/JNEUROSCI.2320-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Vohra BP, Lund FE, Milbrandt J. Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. Journal of Neuroscience. 2009;29:5525–5535. doi: 10.1523/JNEUROSCI.5469-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer H, Oertel WH. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6 hydroxydopamine a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994;59:401–415. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- Sawada H, Ibi M, Kihara T, Urushitani M, Nakanishi M, Akaike A, et al. Neuroprotective mechanism of glial cell line-derived neurotrophic factor in mesencephalic neurons. Journal of Neurochemistry. 2000;74:1175–1184. doi: 10.1046/j.1471-4159.2000.741175.x. [DOI] [PubMed] [Google Scholar]
- Scherman D, Desnos C, Darchen F, Pollak P, Javoy-Agid F, Agid Y. Striatal dopamine deficiency in Parkinson’s disease: role of aging. Annals of Neurology. 1989;26:551–557. doi: 10.1002/ana.410260409. [DOI] [PubMed] [Google Scholar]
- Silva RM, Kuan CY, Rakic P, Burke RE. Mixed lineage kinase-c-Jun N-terminal kinase signaling pathway: A new therapeutic target in Parkinson’s disease. Movement Disorders. 2005a;20:653–664. doi: 10.1002/mds.20390. [DOI] [PubMed] [Google Scholar]
- Silva RM, Ries V, Oo TF, Yarygina O, Jackson-Lewis V, Ryu EJ, et al. CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. Journal of Neurochemistry. 2005b;95:974–986. doi: 10.1111/j.1471-4159.2005.03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PD, Crocker SJ, Jackson-Lewis V, Jordan-Sciutto KL, Hayley S, Mount MP, et al. Cyclin-depen-dent kinase 5 is a mediator of dopaminergic neuron loss in a mouse model of Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13650–13655. doi: 10.1073/pnas.2232515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PD, Mount MP, Shree R, Callaghan S, Slack RS, Anisman H, et al. Calpain-regulated p35/cdk5 plays a central role in dopaminergic neuron death through modulation of the transcription factor myocyte enhancer factor 2. Journal of Neuroscience. 2006;26:440–447. doi: 10.1523/JNEUROSCI.2875-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler RM, Dolcet X, Encinas M, Egea J, Bayascas JR, Comella JX. Receptors of the glial cell line-derived neurotrophic factor family of neurotrophic factors signal cell survival through the phosphatidylinositol 3-kinase pathway in spinal cord motoneurons. Journal of Neuroscience. 1999;19:9160–9169. doi: 10.1523/JNEUROSCI.19-21-09160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan A, Roth KA, Sayers RO, Shindler KS, Wong AM, Fritz LC, et al. In situ immunodetection of activated caspase-3 in apoptotic neurons in the developing nervous system. Cell Death and Differentiation. 1998;5:1004–1016. doi: 10.1038/sj.cdd.4400449. [DOI] [PubMed] [Google Scholar]
- Stefanis L, Burke RE. Transneuronal degeneration in substantia nigra pars reticulata following striatal excitotoxic injury in adult rat: time course, distribution, and morphology of cell death. Neuroscience. 1996;74:997–1008. doi: 10.1016/0306-4522(96)00175-3. [DOI] [PubMed] [Google Scholar]
- Tang X, Wang X, Gong X, Tong M, Park D, Xia Z, et al. Cyclin-dependent kinase 5 mediates neurotoxin-induced degradation of the transcription factor myocyte enhancer factor 2. Journal of Neuroscience. 2005;25:4823–4834. doi: 10.1523/JNEUROSCI.1331-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Yeung J, Lee KY, Matsushita M, Matsui H, Tomizawa K, et al. An isoform of the neuronal cyclin-dependent kinase 5 (cdk5) activator. The Journal of Biological Chemistry. 1995;270:26897–26903. doi: 10.1074/jbc.270.45.26897. [DOI] [PubMed] [Google Scholar]
- Tatton NA, Kish SJ. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labeling and acridine orange. Neuroscience. 1997;77:1037–1048. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
- The Parkinson Study Group PRECEPT Investigators. Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early parkinson disease. Neurology. 2007;69:1480–1490. doi: 10.1212/01.wnl.0000277648.63931.c0. [DOI] [PubMed] [Google Scholar]
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Tibbetts MD, Zheng L, Lenardo MJ. The death effector domain protein family: regulators of cellular home-ostasis. Nature Immunology. 2003;4:404–409. doi: 10.1038/ni0503-404. [DOI] [PubMed] [Google Scholar]
- Timmons S, Coakley MF, Moloney AM, O’Neill C. Akt signal transduction dysfunction in parkinson’s disease. Neuroscience Letters. 2009;467:30–35. doi: 10.1016/j.neulet.2009.09.055. [DOI] [PubMed] [Google Scholar]
- Trupp M, Scott R, Whittemore SR, Ibanez CF. Ret-dependent and -independent mechanisms of glial cell line-derived neurotrophic factor signaling in neuronal cells. The Journal of Biological Chemistry. 1999;274:20885–20894. doi: 10.1074/jbc.274.30.20885. [DOI] [PubMed] [Google Scholar]
- Tsai L-H, Delalle I, Caviness VS, Jr, Chae T, Harlow E. P35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- Tsai L-H, Takahashi T, Caviness VS, Jr, Harlow E. Activity and expression pattern of cyclin-dependent kinase 5 in the embryonic mouse nervous system. Development. 1993;119:1029–1040. doi: 10.1242/dev.119.4.1029. [DOI] [PubMed] [Google Scholar]
- Ugarte SD, Lin E, Klann E, Zigmond MJ, Perez RG. Effects of GDNF on 6-OHDA-induced death in a dopaminergic cell line: modulation by inhibitors of PI3 kinase and MEK. Journal of Neuroscience Research. 2003;73:105–112. doi: 10.1002/jnr.10632. [DOI] [PubMed] [Google Scholar]
- van der Brug MP, Blackinton J, Chandran J, Hao LY, Lal A, Mazan-Mamczarz K, et al. RNA binding activity of the recessive parkinsonism protein DJ-1 supports involvement in multiple cellular pathways. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10244–10249. doi: 10.1073/pnas.0708518105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajant H. Death receptors. Essays in Biochemistry. 2003;39:53–71. doi: 10.1042/bse0390053. [DOI] [PubMed] [Google Scholar]
- Waldmeier P, Bozyczko-Coyne D, Williams M, Vaught JL. Recent clinical failures in parkinson’s disease with apoptosis inhibitors underline the need for a paradigm shift in drug discovery for neurodegenerative diseases. Biochemical Pharmacology. 2006;72:1197–1206. doi: 10.1016/j.bcp.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Wang MS, Fang G, Culver DG, Davis AA, Rich MM, Glass JD. The WldS protein protects against axonal degeneration: a model of gene therapy for peripheral neuropathy. Annals of Neurology. 2001;50:773–779. doi: 10.1002/ana.10039. [DOI] [PubMed] [Google Scholar]
- Xia XG, Harding T, Weller M, Bieneman A, Uney JB, Schulz JB. Gene transfer of the JNK interacting protein-1 protects dopaminergic neurons in the MPTP model of parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:10433–10438. doi: 10.1073/pnas.181182298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahata N, Yuasa S, Araki T. Nicotinamide mono-nucleotide adenylyltransferase expression in mitochondrial matrix delays Wallerian degeneration. Journal of Neuroscience. 2009;29:6276–6284. doi: 10.1523/JNEUROSCI.4304-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Gehrke S, Haque ME, Imai Y, Kosek J, Yang L, et al. Inactivation of drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/akt signaling. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13670–13675. doi: 10.1073/pnas.0504610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Khosravi-Far R, Chang HY, Baltimore D. Daxx, a novel fas-binding protein that activates JNK and apoptosis. Cell. 1997a;89:1067–1076. doi: 10.1016/s0092-8674(00)80294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DD, Kuan CY, Whitmarsh AJ, Rincon M, Zheng TS, Davis RJ, et al. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the jnk3 gene. Nature. 1997b;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Ahuja HS, Zakeri ZF, Wolgemuth DJ. Cyclin-dependent kinase 5 is associated with apoptotic cell death during development and tissue remodeling. Developmental Biology. 1997;183:222–233. doi: 10.1006/dbio.1996.8494. [DOI] [PubMed] [Google Scholar]