In this study, the authors show that propofol, a widely used anesthetic, exerts a marked and selective potentiation on GABAA receptors of retinal bipolar cells.
Abstract
Purpose.
Propofol (2,6-diisopropyl phenol), a widely used systemic anesthetic, is known to potentiate GABAA receptor activity in a number of CNS neurons and to produce changes in electroretinographically recorded responses of the retina. However, little is known about propofol's effects on specific retinal neurons. The authors investigated the action of propofol on GABA-elicited membrane current responses of retinal bipolar cells, which have both GABAA and GABAC receptors.
Methods.
Single, enzymatically dissociated bipolar cells obtained from rat retina were treated with propofol delivered by brief application in combination with GABA or other pharmacologic agents or as a component of the superfusing medium.
Results.
When applied with GABA at subsaturating concentrations and with TPMPA (a known GABAC antagonist), propofol markedly increased the peak amplitude and altered the kinetics of the response. Propofol increased the response elicited by THIP (a GABAA-selective agonist), and the response was reduced by bicuculline (a GABAA antagonist). The response to 5-methyl I4AA, a GABAC-selective agonist, was not enhanced by propofol. Serial brief applications of (GABA + TPMPA + propofol) led to a progressive increase in peak response amplitude and, at higher propofol concentrations, additional changes that included a prolonged time course of response recovery. Pre-exposure of the cell to perfusing propofol typically enhanced the rate of development of potentiation produced by (GABA + TPMPA + propofol) applications.
Conclusions.
Propofol exerts a marked and selective potentiation on GABAA receptors of retinal bipolar cells. The data encourage the use of propofol in future studies of bipolar cell function.
Retinal bipolar cells have GABAA and GABAC receptors, which are pentameric, ligand-gated ion channels activated by γ-aminobutyric acid (GABA). GABAA and GABAC receptors mediate fast inhibitory signaling in bipolar cells of the retina1–3 (see Ref. 4 for review). Electrophysiological and immunocytochemical data indicate that GABAA and GABAC receptors on bipolar cells are localized primarily at the axon terminals, postsynaptic to amacrine cells.5–7 Furthermore, expression of GABAA receptor α1, α3, β2/3, and γ2 subunits and GABAC receptor ρ1 and ρ2 subunits in rod bipolar cells has been reported.8–11 However, the specific subunit compositions of these receptors of bipolar cells are as yet unknown.
A diverse group of chemical compounds is known to affect GABAA receptor activity. These GABAA-modulating agents include benzodiazepines, barbiturates, ethanol, neuroactive steroids, and volatile anesthetics.12,13 Among this extensive group of GABAA modulators is propofol (2,6-diisopropyl phenol), a compound in wide use as a nonvolatile anesthetic14–18 (see Refs. 12, 19 for review). The action of propofol on native and recombinant GABAA receptors of differing subunit composition has been investigated in multiple cell types.20–22 For example, in Xenopus oocytes engineered to express α1β2γ2s GABAA receptors, propofol at low concentrations (∼1–100 μM) allosterically potentiates the GABA-elicited response; the compound at higher concentrations (∼100–1000 μM) activates the receptor in the absence of GABA.22 It has been shown that propofol slows receptor deactivation and desensitization,23 mechanisms that could underlie the enhancement effect of this compound on the GABAA receptor.
The systemic administration of propofol significantly alters electroretinographic (ERG) responses recorded from the intact eye. Kommenen et al.24 tested the effect of increasing the infusion rate of anesthetizing propofol on ERGs recorded from dogs and observed an enhancement of the b-wave response with increasing propofol infusion rate. In addition, Ng et al.25 found that administering propofol to the isolated perfused porcine eye reduces and delays the p1 component of the multifocal ERG. To our knowledge, however, no one has investigated the action of propofol on the responses of an isolated, specified type of retinal neuron.
The major role of bipolar cells in visual signal transmission and processing within the retina and the importance of propofol and related compounds to both fundamental and clinical aspects of GABA receptor physiology raise interest in determining the effects of propofol specifically on GABA receptors of the bipolar cell. The present study was undertaken to address this issue. Preliminary data have been reported (Yue L, et al. IOVS 2009;50:ARVO E-Abstract 1015).
Methods
All animal procedures were conducted in accordance with institutional policies and with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The experiments were conducted on isolated, enzymatically dissociated bipolar cells obtained from adult Sprague-Dawley and Long-Evans rats (male and female) (Charles River Laboratories, Wilmington, MA). Procedures for euthanatization, isolation of the retina, and dissociation of retinal cells were similar to those described by Ramsey et al.26 Isolated bipolar cells were identified on the basis of their morphologic appearance and were likely rod bipolar cells. Whole-cell patch-clamp techniques similar to those described26,27 were used to record membrane current responses to GABA and other test agents. The patch pipette (borosilicate glass capillary) was pulled in two stages using a microelectrode puller (model PP830; Narishige Group, Tokyo, Japan). Unless otherwise indicated, the pipette was filled with an intracellular solution containing 140 mM CsCl, 1 mM CaCl2, 2 mM MgCl2, 11 mM EGTA, and 10 mM HEPES, pH 7.2. Cells were clamped at −60 mV (Axopatch 200B amplifier; Axon Instruments, Union City, CA), and experimental runs were controlled by pCLAMP system software (Axon Instruments). Electrophysiological data were obtained in response to test compounds dissolved in physiological saline (Ringer solution) that consisted of 135 mM NaCl, 5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 10 mM glucose, and 5 mM HEPES, pH 7.4. Test solutions were delivered from separate reservoirs by a multichannel perfusion system. Illustrated stimulus markers are manually positioned to compensate for the dead volume of the perfusion system. Test agents used in the experiments were GABA (Sigma-Aldrich, St. Louis, MO), propofol (SAFC Supply Solutions, St. Louis, MO), 5-methyl-imidazole-4-acetic acid (5Me-I4AA)28, methyl(1,2,5,6-tetrahydropyridin-4-yl)phosphinic acid (TPMPA; Tocris Biosciences, Ellisville, MO), bicuculline (Tocris Biosciences), and 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP; Tocris Biosciences). Supplementation of aqueous test solutions with propofol, bicuculline, and TPMPA was carried out by adding an aliquot of a stock solution containing the compound dissolved in dimethyl sulfoxide (DMSO). In all experiments, the amount of carrier DMSO in the applied test solution was <1% (vol/vol). Unless otherwise indicated, the quoted n number for a given statistical analysis refers to the number of investigated cells. The results reported in this study were obtained from a total of 76 cells.
Noise analysis of representative waveforms obtained in the absence and presence of propofol followed procedures similar to those described.2,26 Responses were recorded at a sampling rate of 2.5 kHz and were segmented into 200-ms intervals. The mean amplitude and variance (calculated after passing a 5-Hz high-pass filter) were determined for each 200-ms segment of the responses. For each investigated cell, data obtained under a given experimental condition were analyzed by linear regression to obtain a value of slope (variance/mean) of the plot of variance versus mean amplitude, and the single-channel conductance was obtained by dividing this ratio by the driving force for chloride ion (60 mV).
Results
Effect of Co-applied Propofol on Responses Elicited by GABA and 5Me-I4AA
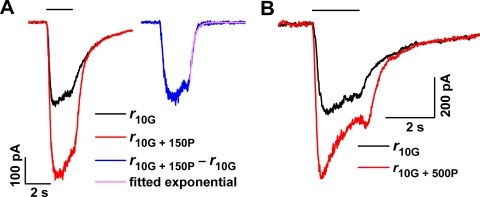
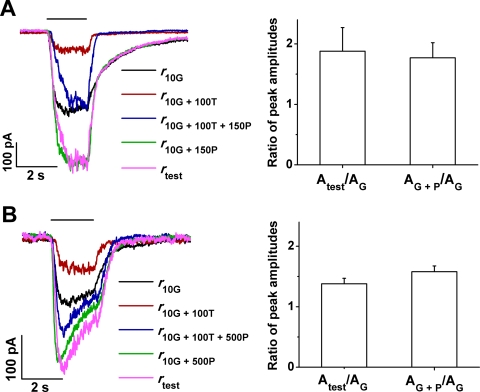
Figure 1, which illustrates responses recorded from a bipolar cell in response to GABA alone and to co-applied GABA and propofol, shows that propofol markedly increased the GABA-elicited response. Shown are responses recorded from a single cell (Fig. 1A), with the application of either 10 μM GABA alone (response r10G; black trace) or co-application of 10 μM GABA plus 150 μM propofol (r10G + 150P; red trace). (Data shown in the inset here are considered later in Results.) Figure 1B shows data from a second cell that received either 10 μM GABA alone or co-applied (10 μM GABA + 500 μM propofol) (black and red traces, respectively). In both experiments, the inclusion of propofol produced a substantial increase in the response (Fig. 1A, 1.8-fold; Fig. 1B, 1.6-fold).
Figure 1.
(A) Responses elicited by 10 μM GABA in the absence (black trace) and presence (red trace) of 150 μM propofol. Inset: difference waveform (blue) and fitted exponential function (purple) for the responses in (A). (B) Effect of co-applied 500 μM propofol on the response to 10 μM GABA.
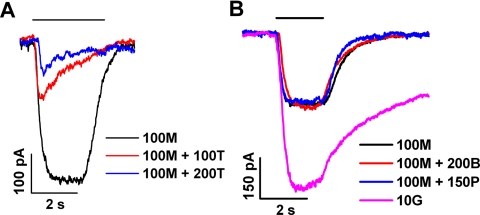
Given that both GABAA and GABAC receptors are present on retinal bipolar cells, we examined the effect of propofol on pharmacologically isolated responses mediated by each receptor type. To activate GABAC receptors, we applied 5Me-I4AA, a recently described GABAC-specific activator that exhibits a micromolar EC50 value at the ρ1 GABAC receptor.28 Figure 2A shows results obtained in a representative experiment that tested the susceptibility of the 5Me-I4AA–mediated response to TPMPA, a known GABAC antagonist. By comparison with the response obtained in the absence of TPMPA (black trace), those obtained with co-applied 100 and 200 μM TPMPA exhibited a dose-dependent, marked reduction in peak amplitude. As shown by the red trace, 100 μM TPMPA, when co-applied with 100 μM 5Me-I4AA, decreased the peak amplitude of the response by approximately 50%, and the diminished response to this co-applied mixture exhibited a pronounced sag from the peak, consistent with a progressively developing inhibition by TPMPA during the period of its application. Figure 2B describes further properties of the 5Me-I4AA response. In this experiment and in six others of similar design, we analyzed the effects of TPMPA (100 μM) and bicuculline (200 μM; a known GABAA antagonist) on the peak amplitude of the response to 100 μM 5Me-I4AA. Here, the choice of 100 μM as the 5Me-I4AA concentration was based on the finding of Madsen et al.28 that, in ρ1-GABAC–expressing HEK 293 cells, the response to 100 μM 5Me-I4AA is below saturation of the 5Me-I4AA response function and amounts to approximately 60% of the response to 10 μM GABA. In the bipolar cell, the peak amplitude of the response to 100 μM 5Me-I4AA represented approximately 50% of the response to 10 μM GABA (black and purple traces, Fig. 2B). Co-application of 200 μM bicuculline did not substantially alter the 5M-I4AA response, as indicated by the red trace in Figure 2B. Results obtained in Figure 2B and the companion experiments are summarized in Table 1, which compares peak amplitudes of responses obtained in the presence versus absence of a given test agent. Together, the data in Figures 2A and 2B and Table 1 indicate sensitivity of the 5Me-I4AA response to TPMPA and insensitivity to bicuculline, consistent with an action of 5Me-I4AA specifically at GABAC receptors.
Figure 2.
Test of propofol activity on responses to 5Me-I4AA. 5Me-I4AA, TPMPA, bicuculline, and propofol are abbreviated in the figure as M, T, B, and P, respectively. (A) Responses obtained from a single cell on the application of 100 μM 5Me-I4AA alone (black trace) and of 100 μM 5Me-I4AA with co-applied 100 or 200 μM TPMPA (red and blue traces, respectively). (B) Responses obtained from a second cell to 100 μM 5Me-I4AA alone (black), 100 μM 5Me-I4AA plus 200 μM bicuculline (red), 100 μM 5Me-I4AA plus 150 μM propofol (blue), and 10 μM GABA alone (purple).
Table 1.
Ratio of Peak Amplitudes Obtained in Experiments Testing 5Me-I4AA and Propofol
| Row | Description* | Value |
|---|---|---|
| 1 | A10G/A100M | 2.15 ± 0.36 |
| 2 | A100M+100T/A100M | 0.50 ± 0.09 |
| 3 | A100M+200B/A100M | 1.04 ± 0.01 |
| 4 | A100M+150P/A100M | 1.01 ± 0.04 |
| 5 | A10G+100T+150P/A10G+100T | 4.57 ± 1.10 |
Values represent mean ± SD of determinations from a total of seven cells, including the cell described in Figure 2B.
Peak amplitudes of response obtained with 10 μM GABA (A10G), 100 μM 5Me-I4AA (A100M), the mixture (100 μM 5Me-I4AA + 100 μM TPMPA) (A100M + 100T), the mixture (100 μM 5Me-I4AA + 200 μM bicuculline) (A100M + 200B), the mixture (100 μM 5Me-I4AA + 150 μM propofol) (A100M + 150P), and the mixture (10 μM GABA + 100 μM TPMPA + 150 μM propofol) (A10G + 100T + 150P).
To examine propofol's action on the GABAC receptors of retinal bipolar cells, we measured the effects of co-applied propofol on 5Me-I4AA–elicited responses of these cells. As shown by the black and blue traces in Figure 2B, the co-application of 150 μM propofol and 100 μM 5Me-I4AA had no substantial effect on the nominal 5Me-I4AA response, indicating that propofol has no effect on the GABAC receptors. We also examined GABAC receptor activity using a combination of 10 μM GABA + 250 μM bicuculline. Under this condition, the addition of 150 μM propofol had relatively little effect on the response obtained (not illustrated).
Effects of Propofol on GABAA Receptors
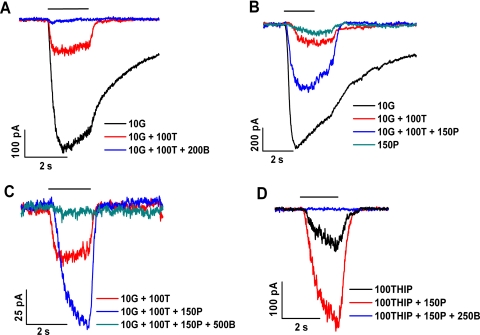
To examine the action of propofol on bipolar cell GABAA receptors, we used TPMPA and bicuculline to suppress, respectively, the contribution of GABAC and GABAA receptors to the overall response elicited by GABA (Fig. 3). Herein GABA, TPMPA, and bicuculline are abbreviated by G, T, and B, respectively, and are accompanied by numbers that refer to the concentration (in μM) of the test agent. (A similar system of abbreviations and concentrations for other test agents will be introduced later in the text.) Figure 3A shows data obtained from a single cell on treatment with these agents. This experiment, and others to be described, used GABA at 10 μM, a concentration that exceeds the EC50 for GABA typically measured for GABAC receptors but below that typically measured for GABAA receptors.29 TPMPA (100 μM), when co-applied with 10 μM GABA, markedly reduced the GABA-elicited response (compare black and red traces in Fig. 3); among 10 cells, including those described in Figure 3A, the reduction produced by 100 μM TPMPA was 83% ± 2% (mean ± SD). Application of the mixture (10 μM GABA + 100 μM TPMPA), that is, (10G + 100T), was used as the nominal condition with which to compare the effects of propofol on bipolar cell GABAA receptors.
Figure 3.
Potentiating effect of propofol on the GABA-elicited response. (A–D) Results obtained from four different cells. The key in each panel describes the temporal order (top to bottom) of the test applications. (A) 10 μM GABA (10G; black); 10 μM GABA plus 100 μM TPMPA (10G + 100T; red); 10 μM GABA plus 100 μM TPMPA plus 200 μM bicuculline (10G + 100T + 200B; blue). (B) 10 μM GABA (10G; black); 10 μM GABA plus 100 μM TPMPA (10G + 100T; red); 10 μM GABA plus 100 μM TPMPA plus 150 μM propofol (10G + 100T + 150P; blue); and 150 μM propofol (150P; green). (C) 10 μM GABA plus 100 μM TPMPA (10G + 100T; red); 10 μM GABA plus 100 μM TPMPA plus 150 μM propofol (10G + 100T + 150P, blue); and 10 μM GABA plus 100 μM TPMPA plus 150 μM propofol plus 500 μM bicuculline (10G + 100T + 150P + 500B; green). (D) 100 μM THIP (100THIP; black); 100 μM THIP plus 150 μM propofol (100THIP + 150P; red); and 100 μM THIP plus 150 μM propofol plus 250 μM bicuculline (100THIP + 150P + 250B; blue).
When co-applied with (10G + 100T), propofol at a concentration of 150 μM (i.e., 150P) markedly increased the response (Fig. 3B, blue trace). Among data obtained in the experiment depicted in Figure 3B and in five similar experiments, the ratio of the peak amplitude of the response to (10G + 100T + 150P propofol) compared with the response to (10G + 100T) was 5.8 ± 2.3. Henceforth, the ratio describing the propofol-induced increase in peak amplitude will be termed the “enhancement” factor for the response. It should be noted that the size of the propofol-dependent increase in the peak amplitude of the response typically exhibited a progressive change with repeated application of the propofol-containing test mixture. Throughout the present study, calculation of the increase in peak amplitude caused by propofol application was based on use of the maximum response recorded among what typically was a group of ≥3 responses obtained at a given propofol concentration.
Figure 3B also illustrates the response to 150 μM propofol alone (green trace) and shows that the peak amplitude of this response is small, similar to that produced by (10G + 100T). Accordingly, for simplicity in the following sections, we refer to the peak amplitude enhancement factor described above as indicating the “potentiating” effect of propofol. In other words, we ignore the small contribution due to propofol's agonist activity. Figure 3C shows results obtained in another experiment that tested the effect of bicuculline on the propofol-enhanced response. Here, the response to (10G + 100T) (red trace) was enhanced by co-application of 150 μM propofol (10G + 100T + 150P) (blue trace), and supplementation of the propofol-containing mixture with 500 μM bicuculline (10G + 100 T + 150P + 500B) led to almost complete inhibition of the propofol-enhanced response (green trace). In other experiments (not shown) conducted with 100 μM rather than 500 μM bicuculline, we found that this lower concentration of bicuculline inhibits, by 56% ± 8% (n = 4), the response elicited by (10 μM GABA + 100 μM TPMPA + 150 μM propofol).
The enhancement of GABAA receptor activity on retinal bipolar cells is further indicated by the response elicited by THIP, a GABAA-selective partial agonist. As determined in the cell described in Figure 3D and in three others, the co-application of 150 μM propofol and 100 μM THIP (100THIP + 150P) increased the response elicited by 100 μM THIP alone (100THIP) by 5.8 ± 1.1-fold (compare black and red traces in Fig. 3). Further supplementation with 250 μM bicuculline essentially fully eliminated the response to (100THIP + 150P; blue trace).
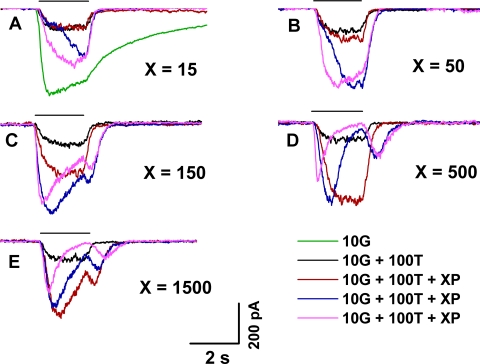
Effect of Repeated Propofol Applications
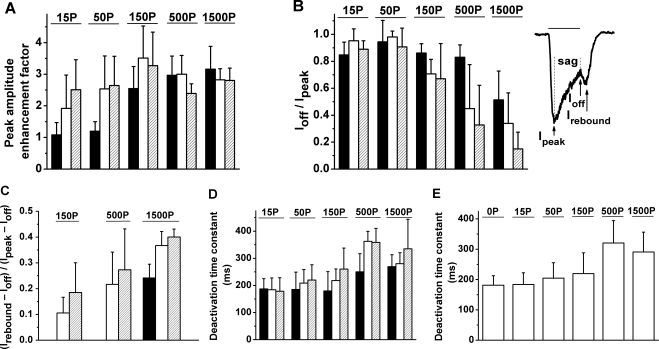
The effects of propofol typically were not fully expressed on an initial brief exposure to propofol. Rather, repeated exposure of the cell to propofol led to progressive changes in the shape of the elicited current response. Figure 4 describes these progressive changes in relation to the concentration of applied propofol. Shown are results obtained in a single representative experiment when a mixture consisting of 10G, 100T, and a varying concentration of propofol was repeatedly applied at intervals of ∼15–25 seconds (Figs. 4A–E). At low concentrations of propofol (15 and 50 μM; Figs. 4A, 4B), initial application of the propofol-supplemented mixture (red trace) produced relatively little change from the nominal response to (10G + 100T) (black trace), but a large increase in peak amplitude (i.e., a response enhancement) developed with repeated presentation of the mixture (blue and purple traces, sequentially). Accompanying this change was an acceleration of the response's rising phase. By contrast, at the higher propofol concentrations (500 and 1500 μM; Figs. 4D, 4E), propofol's potentiating effect was essentially fully exhibited on the first presentation of the mixture (red trace). Here, repeated presentations led to a progressive decrease in peak amplitude from that expressed with the first propofol application, and to an increasingly pronounced sag from peak as well as a substantial rebound on termination of the applied mixture (blue and purple traces). Responses obtained with an intermediate propofol concentration (150 μM; Fig. 4C) showed a pattern intermediate between those exhibited at the lower and higher propofol concentrations. In the experiment of Figure 4, the data shown in Figures 4A–E were collected consecutively; that is, the groups of repeated exposures to single concentrations of propofol were conducted in sequential (A-to-E) order. The (10G + 100T) response was collected as a nominal response immediately before each group of test applications involving propofol. The observed similarity of these (10G + 100T) responses indicated that there was little, if any, post-potentiating effect of propofol under the investigated conditions (i.e., with ∼15–25 seconds of continuous Ringer perfusion between tests).
Figure 4.
Effect of repeated applications (2 seconds in duration) of propofol-containing test mixtures. Responses obtained with the use of 15, 50, 150, 500, and 1500 μM propofol (A–E, respectively). Waveforms within each panel show the nominal response to (10 μM GABA + 100 μM TPMPA) (black) and responses to a subsequent series of test applications in which a fixed concentration of propofol supplemented the (10 μM GABA + 100 μM TPMPA) solution (red, blue, purple). All data were obtained from the same cell (temporal order A–E), and waveforms within each panel were obtained in the top-to-bottom temporal order shown in the key. The response of the cell to 10 μM GABA alone is shown in (A) (green). Intervals between successive test applications (periods of perfusion with unsupplemented Ringer) were ∼15–25 seconds long.
Figure 5 illustrates combined results obtained from four cells in experiments of the type described in Figure 4. Figure 5A shows that at lower concentrations of propofol (15–50 μM), the enhancement factor produced by the third application significantly exceeded that produced by the first application (P = 0.04 for 15 μM; P = 0.02 for 50 μM). By contrast, at higher propofol concentrations (150–1500 μM), the enhancement factor produced by the successive applications of the mixture did not significantly differ from that produced by the first application (P = 0.10 for 150 and 1500 μM; P = 0.06 for 500 μM). Figure 5B shows results obtained for the amplitude of the response at conclusion of the application (Ioff), compared with the response's peak amplitude (Ipeak). At low concentrations of propofol (15–50 μM), the peak of the response occurred at or shortly before termination of the application, and the ratio Ioff/Ipeak was near unity for all three applications. At 150 and 500 μM propofol, there was little sag in the initially recorded response (Ioff/Ipeak near unity), but a progressively developing sag was evident in the second and third responses. At 1500 μM propofol, considerable sag was present even in the first response. The relatively large SD evident under several conditions at intermediate and high propofol concentrations indicates variation among cells in the relative extent of the sag. Figure 5C further describes the rebound component of the response seen at the intermediate and high propofol concentrations (see Fig. 4). To quantify the size of this rebound component, both the absolute peak amplitude of the response (Ipeak) and the peak of the rebound component (Irebound) were referenced to Ioff, and the value of the ratio (Irebound − Ioff)/(Ipeak − Ioff) was determined. At each of the three propofol concentrations for which a substantial rebound was observed, the average value of (Irebound − Ioff)/(Ipeak − Ioff) produced by the second application exceeded that produced by the first application, and at 1500 μM propofol this difference was significant (P = 0.02). At all concentrations except 1500 μM propofol, the response to the first application lacked a rebound component. In addition, the large standard deviations observed with 150 μM and 500 μM propofol indicated variation in the concentration threshold for appearance of the rebound component. This rebound component was evident in all cells tested at 1500 μM propofol; thus, the SD at this highest concentration was relatively small.
Figure 5.
Analysis of responses to three successive applications of test mixtures that contained 10 μM GABA, 100 μM TPMPA, and a defined concentration of propofol ranging from 15 to 1500 μM. Aggregate data obtained from a group of cells including that described in Figure 4. Each group of three histograms in (A–D) indicate the mean ± SD of a given response property determined, respectively, for the first (filled bar), second (open bar), and third (striped bar) application. Horizontal bar above each group of histograms: propofol concentration in μM. Data obtained from four to five cells at a given propofol concentration. (A) Propofol-induced enhancement factor (ratio of peak amplitudes). (B) Ioff/Ipeak, a measure of response sag. (C) (Irebound − Ioff)/(Ipeak − Ioff), a measure of response rebound. Ipeak, Ioff, and Irebound for a representative single waveform [response to (10G + 100T + 150P)] are illustrated at the right in (B). (D) Deactivation time constant after test mixture application. (E) Combined results for deactivation time constant obtained from the first, second, and third applications at a given propofol concentration. Histogram 0P: data obtained with omission of propofol [i.e., responses to (10G + 100T)].
Figure 5D shows determinations of the deactivation time constant of the response to the test applications described in the preceding paragraphs. Responses to the first, second, and third applications obtained under a given experimental condition were analyzed by fitting a simple exponential decay function to the recovery phase of the response (i.e., the waveform exhibited during wash-out of the applied GABA, TPMPA, and propofol). The deactivation time constants determined with 15 to 50 μM propofol did not exhibit a significant change with repeated propofol applications (P = 0.60 for 15 μM; P = 0.15 for 50 μM). Aggregate average values of these time constants (i.e., average combined results for the first, second, and third applications) obtained with 15 and 50 μM propofol applications were 184 ± 39 ms and 205 ± 51 ms, respectively. These values did not differ significantly from one another (P = 0.32; n = 12), nor did they differ significantly from the time constant determined in the absence of propofol (181 ± 31 ms; P = 0.44 for 15 μM; P = 0.10 for 50 μM; Figure 5E). At higher propofol concentrations, there was a progressive increase in the average time constant associated with the first application, and, at a given propofol concentration, repeated application of the propofol-containing mixture produced a further increase in the average time constant. Aggregate values of the time constants determined at 500 and 1500 μM propofol differed significantly from those determined in the absence of propofol (P ≤ 10−5); there was no significant difference between the aggregate value obtained at 150 μM and that obtained at 0 μM propofol (P = 0.07).
The experiments described above used 10 μM GABA (in combination with 100 μM TPMPA), the response to which was much smaller than the saturating GABA response. We used a protocol similar to that depicted in Figure 4 to determine whether propofol potentiation is exhibited at 30 μM GABA and whether the effects of propofol on the shape and deactivation time constant of the response at 30 μM GABA resemble those observed at the lower GABA concentration. Here the concentration of 30 μM was chosen based on the approximate correspondence of this concentration with the EC50 for GABA at α1β2γ2 GABAA receptors. Under this condition, the potentiating effect of 150 μM propofol on the response to (30G + 200T) was smaller than that exhibited at 10 μM GABA, and the first of three serial responses exhibited the greatest amplitude (average enhancement factor, 1.3 ± 0.1; n = 3). The responses to later applications showed, on average, a progressively more extensive sag and a relatively more pronounced rebound current. Specifically, the average Ioff/Ipeak obtained for the first, second, and third applications, respectively, was 0.72, 0.60, and 0.56, and the average (Irebound − Ioff)/(Ipeak − Ioff) was 0.06, 0.19, and 0.20, respectively. As in the case of 10 μM GABA (Fig. 5D), treatment with propofol increased the deactivation time constant from 122 ± 27 ms to 335 ± 120 ms (P = 0.0005).
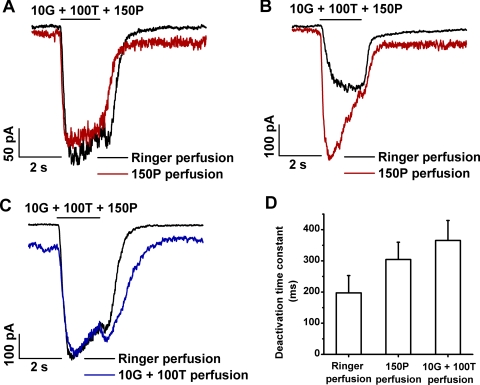
To elucidate the nature of the progressive changes in the (10G + 100T + propofol) response described by Figures 4 and 5, we conducted experiments in which applications of GABA and TPMPA were superimposed on a continuous background of perfusion with propofol-containing medium. We also conducted a complementary type of experiment in which medium containing (10G + 100T) was substituted for Ringer perfusion. Figure 6 shows results obtained in these experiments with altered perfusing medium. Figures 6A and 6B show results of two representative experiments, each of which involved determinations of the response to (10G + 100T + 150P) without versus with the inclusion of 150 μM propofol in the perfusion medium. In each experiment, the response obtained with Ringer perfusion shown by the black trace was the “stabilized” response of the cell to (10G + 100T + 150P); this response was exhibited in the fourth or fifth experimental run among a series of (G + T + P) applications and showed no substantial difference from the preceding run (i.e., ≤10% change in peak amplitude between consecutive runs). In the experiments depicted in Figures 6A and 6B, the enhancement exhibited by the stabilized (10G + 100T + 150P) response, relative to that of the (10G + 100T) response, did not differ substantially (data not shown). However, the nominal (10G + 100T + 150P) responses obtained in the two experiments exhibited differences in both the rate of response onset and the relative extents of sag and rebound current. These differences are consistent with the finding, in the experiments depicted in Figures 5B and 5C, of substantial variation (i.e., relatively large SD) in the extents of response sag and rebound component [Ioff/Ipeak and (Irebound − Ioff)/(Ipeak − Ioff), respectively] among later responses in series of experimental runs with ≥150 μM propofol. Thus, for example, the stabilized nominal response (black trace) of Figure 6A exhibits a relatively fast onset and the presence of a small rebound, whereas that of Figure 6B has a relatively slow onset and no rebound current. Red traces in Figures 6A and 6B show the response to (100G + 100T + 150P) obtained with propofol perfusion. In both experiments, the perfusion with propofol-containing medium was initiated shortly after recording of the nominal response (black trace), and the initiation of propofol perfusion corresponded with the start of recording of the red trace. In the Figure 6A experiment, the red trace closely resembled the nominal response during the period of (10G + 100T + 150P) application but, unlike the nominal response, lacked a rebound component and exhibited a prolonged deactivation phase at the conclusion of the test mixture application. In the Figure 6B experiment, the (10G + 100T + 150P) response obtained with propofol perfusion exhibited a rapid rise to peak and a pronounced subsequent sag as well as a prolonged deactivation phase.
Figure 6.
Dependence of the response to (GABA + TPMPA + propofol) (G + T + P) on perfusion with propofol-containing medium or (G + T)-containing medium. (A) Responses obtained in a single representative experiment before (black) and during (red) perfusion with medium that contained 150 μM propofol. (B) Responses obtained from another cell before (black) and during (red) propofol perfusion. In the experiments of (A) and (B), the switch to propofol-containing medium occurred at the beginning of the recording of the red trace. (C) Responses obtained from a cell before (black) and during (blue) perfusion with 10 μM GABA plus 100 μM TPMPA (10G + 100T). The switch to (10G + 100T)-containing medium occurred at the beginning of the recording of the blue trace. (D) Deactivation time constants determined for perfusion with Ringer, propofol, and (G + T). Histograms show the mean ± SD for data obtained from five cells.
Figure 6C compares the stabilized response to (10G + 100T + 150P) (black trace) with the response obtained when the perfusing medium was supplemented with (10G + 100T) (blue trace). Here, the response to the (10G + 100T + 150P) in the (10G + 100T)–containing medium departed from a level that itself reflected activation by the perfusing (10G + 100T) (compare baselines of the two illustrated traces). Furthermore, by comparison with the nominal stabilized response, that obtained with (10G + 100T) perfusion showed a similar onset rate and peak amplitude (including the baseline difference) and a somewhat enhanced rebound current.
Figure 6D describes the effect of perfusion with propofol-containing medium and with (10G + 100T)–containing medium on the deactivation time constant of the response to applied test mixture containing (10G + 100T + 150P). As in the experiments depicted in Figure 5D, exponential time constants were determined for the recovery phase of the (10G + 100T + 150P) response. Relative to the time constant determined with Ringer perfusion, perfusion with 150 μM propofol or with (10 μM GABA + 100 μM TPMPA) significantly increased the time constant [P = 0.00004 for 150P perfusion; P = 0.001 for (10G + 100T) perfusion].
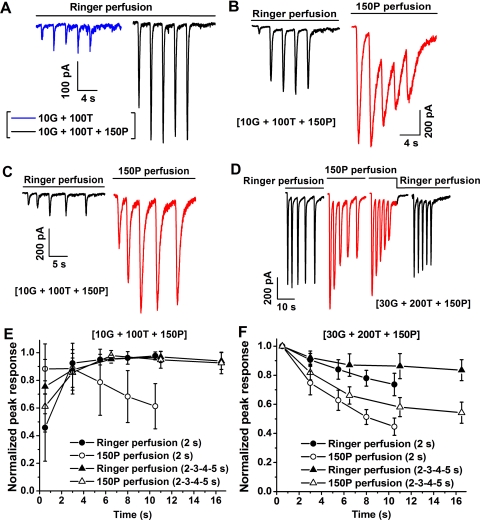
In the experiments depicted in Figures 7A–C, we examined the effect of propofol perfusion on the rising-phase kinetics of responses to serial applications of (10G + 100T + 150P) by determining the amplitude of the response at the conclusion of each of a series of brief (500-ms) applications. The period of 500 ms is comparable to or shorter than the time-to-peak of the (10G + 100T + 150P) response (Fig. 4). Thus, an effect of propofol perfusion on the initial 500-ms portion of the response's rising phase should be evident as a change in the response amplitude determined at the end of the application. To consider the effect of propofol observed in this type of experiment, it is helpful to consider first the results obtained in a control experiment in which repeated applications of (10G + 100T) (Fig. 7A, blue trace) and (10G + 100T + 150P) (Fig. 7A, black trace) were presented during perfusion with Ringer. (Here and in Figs. 7B–D, the composition of the test mixture is shown in brackets.) For both test mixtures, responses were obtained to a series of five applications presented at fixed intervals (2 seconds). As in the experiments depicted in Figure 4, inclusion of 150 μM propofol in the applied test mixture (black trace) markedly enhanced the peak amplitude of the response, and the first response to G + T + P exhibited only partial development of the propofol-induced enhancement of the peak amplitude. Figure 7B shows results of a second experiment that examined responses to (10G + 100T + 150P) obtained with Ringer perfusion (black trace) and, subsequently, with perfusion medium that contained 150 μM propofol (red trace). This change in perfusion medium altered the response to (10G + 100T + 150P). Specifically, the exposure to perfusing propofol substantially increased the peak amplitude of the first response to (10G + 100T + 150P), and responses to late applications in the series exhibited reduced peak amplitude. Figure 7C shows data from an experiment similar to that of Figure 7B, but in which, for both Ringer perfusion (black trace) and propofol perfusion (red trace), the test mixture of (10G + 100T + 150P) was delivered at increasing inter-application time intervals ranging from 2 to 5 seconds. This lengthening of interval between successive applications preserved a substantial initial increase in peak amplitude produced by propofol perfusion but diminished the subsequent decline in peak amplitude. The interplay of the effects of propofol perfusion and of the timing of (G + T + P) applications is further described by the results shown in Figure 7D. The design of the Figure 7D experiment was generally similar to that shown in Figures 7B and 7C; here, however, the (G + T + P) test mixture contained 30 μM GABA and 200 μM TPMPA in addition to 150 μM propofol. The 30-μM concentration of GABA was used to achieve a nominal condition of relatively fast onset of the propofol-potentiated response and a reduced nominal extent of potentiation. The initial phase (initial black trace) of the experiment of Figure 7D involved Ringer perfusion and increasing inter-application intervals (2–5 seconds) between (G + T + P) applications. This initial phase was followed, sequentially, by phases (red traces) that involved propofol perfusion with 2- to 5-second inter-application intervals, propofol perfusion with fixed (2-second) inter-application interval, and Ringer perfusion with 2-second inter-application interval. As in Figures 7B and 7C, the fixed, relatively short interval between (G + T + P) applications in propofol perfusion produced a relatively more extensive progressive decline in peak amplitude of the (G + T + P) response. Thus, with propofol perfusion, the frequency of test mixture application, and not merely the duration of exposure to propofol, affects the extent of decline in peak amplitude of the (G + T + P) response. The pattern of data obtained in these experiments with propofol perfusion is further described by Figure 7E and 7F, which show aggregate results obtained in experiments of the types described in Figures 7B and 7C (n = 7) and Figure 7D (n = 7), respectively.
Figure 7.
Response series obtained with Ringer versus propofol perfusion. (A) Responses to 500-ms applications of (10G + 100T) (blue) and of (10G + 100T + 150P) (black) obtained in a single experiment. (B) Responses to 500-ms applications of (10G + 100T + 150P) (interval between applications: 2 seconds) obtained with Ringer perfusion (black) and propofol perfusion (red); initiation of propofol perfusion coincided with initiation of recording of the red trace. Data obtained from a single cell. (C) Conditions as in (B) except with increasing interval between applications of (10G + 100T + 150P). Data obtained from a single cell. (D) Conditions as in (B) and (C) except with increased concentration of GABA (30 μM) and TPMPA (200 μM). Data obtained from a single cell. (E, F) Aggregate data (mean ± SD) obtained in experiments of the types described in (B) and (C), and in (D), respectively. Each family of data represents results obtained from five to seven cells. For each experimental protocol described in the key, maximal amplitudes of the five successive responses are normalized to the greatest maximal amplitude within the series.
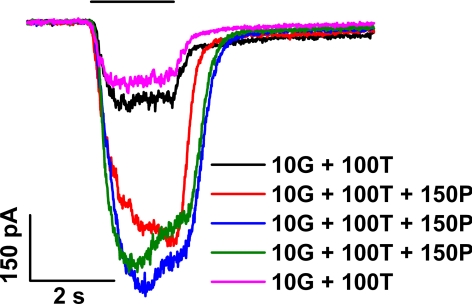
We considered the possibility that the effect of propofol depends on an action of the compound at or near the intracellular side of the cell membrane. For example, the gradual development of the changes seen with repeated applications of propofol might reflect the time required for diffusion of the lipophilic, externally applied propofol across the membrane. To test this possibility, we asked whether the inclusion of propofol in the solution contained within the patch pipette, a condition expected to facilitate the passage of propofol to the interior of the cell, alters the response to either the nominal (10G + 100T) test solution or to a propofol-supplemented test solution (10G + 100T + 150 P). Figure 8 shows results obtained in a single representative experiment that used supplementation of the standard pipette solution (see Methods) with 200 μM propofol. As illustrated by the black trace recorded immediately after breakage of the patch membrane and by the purple trace recorded at ∼1.5 minutes after membrane breakage, there was no substantial change of either the peak amplitude or the waveform of the (10G + 100T) response. (These traces are comparable to those recorded without propofol in the pipette, for example, with the black traces in Fig. 4 obtained under otherwise identical conditions.) Furthermore, successive applications of (10G + 100T + 150 P) produced changes in response size and kinetics similar to those observed in the Figure 4 experiment (compare the red, blue, and green traces in Fig. 8 with, respectively, the red, blue, and purple traces in Fig. 4C). In addition, as determined in the Figure 8 experiment and in four others of identical design, the enhancement factor resulting from application of the (10G + 100T + 150P) test solution was 5.6 ± 2.9, which did not differ significantly from the factor determined with use of the standard pipette solution (P = 0.92).
Figure 8.
Responses obtained with the inclusion of propofol in the solution filling the patch pipette. Data obtained from a single cell. Test applications consisted of (10G + 100T) (black, purple) and (10G + 100T + 150P) (red, blue, green).
Noise Analysis
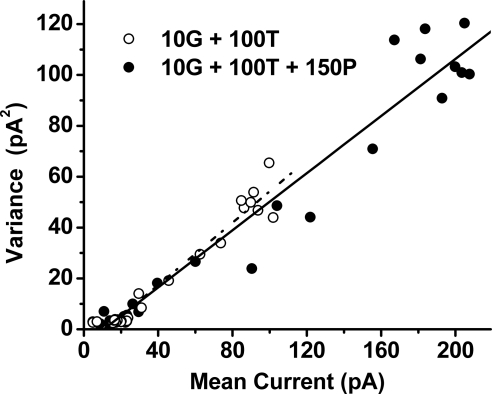
The increase in response size induced by propofol (Figs. 3 and 4) could reflect an increase in single-channel conductance, an increased probability of channel opening by GABA, or some combination of these two effects. To investigate this issue, we analyzed responses from a group of cells to determine the single-channel conductance in the absence versus the presence of propofol. As in the experiments depicted in Figures 3 and 4, we used treatment with 10 μM GABA plus 100 μM TPMPA as the nominal conditions for investigating the effect of propofol. Figure 9 shows the results of analysis of waveforms recorded from a single cell on the application of (10G + 100T) (open circles) and of (10G + 100T + 150P) (filled circles). Determinations of the mean and variance of the analyzed responses over 200-ms intervals (see Methods) yielded slope values of 0.61 pA and 0.56 pA for the data obtained with (10G + 100T) and (10G + 100T + 150P), respectively. The resultant respective values for single-channel conductance were 10.2 and 9.4 pS. Among a group of nine cells, including that described in Figure 9, mean values of single-channel conductance determined with (10G + 100T) were 11.8 ± 3.95 pS, and those determined with (10G + 100T + 150P) were 12.8 ± 4.49 pS. The ratio of single-channel conductances obtained in the presence versus the absence of propofol—i.e., the conductance determined with (10G + 100T + 150P) divided by that determined with (10G + 100T)—yielded 1.1 ± 0.18. Thus, 150 μM propofol had no significant effect on the single-channel conductance (P = 0.19).
Figure 9.
Comparative noise analysis of responses to (10G + 100T) (open circles) versus (10G + 100T + 150P) (filled circles). Representative data obtained from a single cell; data shown by a given symbol type represent results obtained from analysis of a single waveform.
Deactivation Kinetics of the GABA-Elicited Response
Previous studies have described distinct differences in the kinetics of recovery of the GABA-elicited responses of GABAA and GABAC receptors. Specifically, after the cessation of GABA application, the rate of recovery to baseline exhibited by GABAA receptors typically exceeds the recovery rate exhibited by GABAC receptors.27,30 To examine the recovery kinetics of the response component that is potentiated by propofol, we analyzed the responses shown in Figure 1 to obtain a difference waveform that represented the propofol-enhanced component. The inset in Figure 1A illustrates the computational operation that was performed. Specifically, in the experiment depicted in Figure 1A, we determined the difference between the black waveform (GABA-alone response) and the red waveform [(GABA + propofol) response]; the difference is shown by the blue waveform in the Figure 1A inset. For times t ≥ t1, this difference waveform was analyzed through the relation (purple curve, Fig. 1A inset)
where time t1 represents the beginning of the analyzed recovery interval, Idiff(t) is the measured amplitude of the difference waveform at time (t), B and C are constants, and τdiff is a decay time constant. An analysis of this type was carried out on data obtained from five cells including that described in Figure 1A. In all cases, the time chosen as the beginning of the analyzed interval (t1) was within 300 ms of conclusion of the test mixture application, and the length of the investigated interval was ≥4 seconds. Data obtained from these five cells yielded τdiff = 219 ± 70 ms. This time scale is short by comparison with the overall recovery period of both the GABA-alone and (GABA + propofol) responses and is consistent with the time scale of GABAA deactivation under conditions of treatment with 150 μM propofol (219 ± 69 ms; Fig. 5E).
Test of Independence of GABAA- and GABAC-Mediated Responses
As a quantitative test of whether coactivation of the bipolar cell's GABAA and GABAC receptors affects the potentiation by propofol, we examined the relationship of responses obtained with and without selective, TPMPA-induced suppression of the GABAC-mediated component. We reasoned that if propofol affects only the GABAA-mediated component of the overall response to (GABA + propofol) and if this overall (GABA + propofol)-elicited response represents the independent summation of GABAA- and GABAC-mediated components, the sum of the putative GABAC-isolated response plus the propofol-enhanced response obtained under conditions of GABAC suppression should approximate the overall response to (GABA + propofol). To test this hypothesis, we analyzed responses obtained under four experimental conditions: (1) 10 μM GABA alone (10G), (2) 10 μM GABA plus 100 μM TPMPA (10G + 100T), (3) 10 μM GABA + 100 μM TPMPA + 150 μM propofol (10G + 100T + 150P), and (4) 10 μM GABA + 150 μM propofol (10G + 150P). The left side of Figure 10A shows responses obtained in a single experiment of this type. Here the black, red, and blue traces were obtained under conditions 1, 2, and 3, respectively. With the use of these responses, we computationally determined response rtest(t), where
and where [r10G(t) − r10G+100T(t)], representing the difference between the response to GABA alone and the GABAC-suppressed and thus solely GABAA-mediated response to GABA plus TPMPA, is the putative GABAC-mediated component; and where r10G+100T+150P(t) is the putative propofol-enhanced, GABAA-mediated component. On the hypothesis presented, rtest(t) should correspond with the overall response to GABA plus propofol exhibited in the absence of GABAC suppression by TPMPA; that is,
where r10G+150P(t) is the overall response to GABA plus propofol obtained under condition 4 (green trace). As illustrated by the purple and green traces in Figure 10A, the computationally determined waveform rtest(t) (purple) closely resembles r10G+150P(t) (green). In similar experiments we analyzed responses obtained under conditions 1–4, but with a propofol concentration of 500 μM rather than 150 μM (Fig. 10B). As shown in the left side of Figure 10B, again the computationally determined response rtest(t) approximated response r10G+150P(t). Histograms at the right in Figures 10A and 10B describe aggregate results obtained in a group of experiments of the types described by the waveforms in the respective panels. Here, responses obtained under the experimental conditions 1–4 were analyzed to determine the respective peak amplitudes A10G, A10G+100T, A10G+100T+150P, and A10G+150P, and the test peak amplitude Atest, where (equation 2)
With these peak amplitudes, and with the use of A10G as a normalizing factor, we tested for an equality of the dimensionless expression
The histograms in Figures 10A and 10B compare determinations of the left-hand and right-hand expressions of equation 3b under conditions involving treatment with 150 μM and 500 μM propofol, respectively. For 150 μM propofol, values obtained for the left-hand expression (1.88 ± 0.39; data obtained from six cells) did not differ significantly from those for the right-hand expression (1.77 ± 0.25) (P = 0.57), and the difference on average was only 6%. Similarly, for 500 μM propofol, determinations for the equation 3b left-hand expression (1.38 ± 0.09; data obtained from five cells) did not differ substantially from those for the right-hand expression (1.55 ± 0.06) (P = 0.05), and the difference was on average 11%. Together, the Figure 10 data are consistent with an approximate equality of the left- and right-hand expressions of equation 3b and, thus, with an independence of GABAC- and enhanced GABAA-mediated contributions to the overall response to GABA.
Figure 10.
Test of the hypothesis of independent GABAC-mediated and propofol-enhanced GABAA-mediated contributions to the overall GABA-elicited response. (A, B) Comparison of the response to (10 μM GABA + propofol) (green) with rtest, a computationally derived response that represents the summed, hypothetically independent contributions of GABAA- and GABAC-mediated responses (purple). Results obtained with propofol concentrations of 150 μM (A) and 500 μM (B). (A, B) Keys identify the responses leading to the determination of rtest (equations 2a, 2b). Histograms at the right in each panel: ratios of peak amplitudes (equations 3a, 3b) that represent the test of independence at 150 and 500 μM propofol, respectively.
Discussion
Evidence for GABAA Potentiation
The results provide direct evidence for a potentiating effect of propofol on the GABA-elicited response of retinal bipolar cells. Six types of evidence lead us to conclude that this action of propofol is mediated largely or entirely by the GABAA receptors of the bipolar cell. First, propofol potentiates the GABA response obtained in the presence of the GABAC antagonist TPMPA (Figs. 3, 4). Second, this response is virtually entirely eliminated by the GABAA antagonist bicuculline (Fig. 3C). Third, propofol also potentiates the response to THIP, a compound that on retinal bipolar cells activates only GABAA receptors (Fig. 3D). Fourth, propofol does not enhance the response to 5Me-I4AA, a GABAC-selective agonist (Fig. 2). The fifth type of evidence concerns the deactivation kinetics of the response to co-applied 10 μM GABA and 150/500 μM propofol (Fig. 1). By contrast with the response to 10 μM GABA alone, which is dominated by a slow deactivation phase (thus largely reflecting GABAC activity), the response to (10G + 150/500P) exhibits, in addition, a pronounced fast phase consistent with the relatively fast deactivation kinetics of GABAA receptors. Sixth, with respect to both waveshape and peak amplitude, the computationally determined, propofol-potentiated test response [rtest(t) and Atest, respectively] approximates the propofol-potentiated response obtained in the absence of TPMPA (Fig. 10).
The results presented in Figures 3 and 4 indicate that, under the present experimental conditions using 10 μM GABA as a nominal agonist concentration, 100 μM TPMPA suppresses, on average, 83% of the GABA response. Thus, the bipolar cell GABAA receptors contribute only a minor fraction (∼17%) of the overall GABA-elicited response. This observation is consistent with results obtained by Euler and Wässle,6 who isolated GABAA–mediated responses with 3-aminopropyl-(methyl)phosphinic acid (3-APMPA), a GABAC antagonist, and examined GABA-elicited compared with (GABA + 3-APMPA)-elicited responses of rod bipolar cells in the rat retinal slice preparation. Euler and Wässle6 furthermore tested the GABAC contribution to the total GABA response at different GABA concentrations and found that, at 5 μM GABA, 100% of the response is GABAC-mediated; the GABAC contribution decreased to 60% at 25 μM GABA and to 50% at 125 μM GABA. Euler and Wässle6 noted that the relatively high EC50 value of the GABAA receptor and the low EC50 value of the GABAC receptor are the likely basis of the increased GABAA contribution and the decreased GABAC contribution observed with increasing GABA concentration.
Preservation of Nominal Single-Channel Conductance
In previously studied systems, the effect of propofol on the GABA response function has been observed to reflect an increased sensitivity of the receptor to agonist rather than to an increase in the maximal, saturating membrane current.16 On this basis, the potentiating effect of propofol has been attributed primarily to an action of the compound in increasing the probability of channel opening as opposed to an increase in the conductance of individual channels. Consistent with this finding and interpretation, our results obtained with 10 μM GABA and 30 μM GABA show a decreased potentiation factor associated with an increased GABA concentration. In principle, determining whether propofol increases GABAA single-channel conductance in the bipolar cell could come from single-channel recording of propofol's effect when co-applied with GABA. However, we found such a measurement to be difficult in the present system. In the present Figure 9 experiments, we analyzed response noise to derive values for single-channel conductance in a response regime well below saturation. Under these conditions, propofol at 150 μM, a concentration with substantial potentiating activity, produced little, if any, change in the single-channel conductance of the (presumably GABAA) receptors that mediate the propofol-dependent increase. We thus conclude that, as in other cell types,16 the mechanism of propofol potentiation of bipolar cell GABAA receptors is unlikely to involve an increase in single-channel conductance.
Progressive Development of the Effects of Propofol
The experiments depicted in Figures 4 and 5, which involved repeated brief applications of propofol with approximately 15- to 25-second intervals between applications, reveal two prominent features of propofol's action at bipolar cells. First, at midrange or high concentrations, the action of propofol extended beyond merely the enhancement effect discussed above (i.e., beyond merely an increase in the peak amplitude of the response). That is, the fully developed waveshape also includes a relatively rapid onset rate, a prominent sag after the peak, and a rebound current on the cessation of (G + T + P) application. Second, the potentiating and other effects of propofol typically were not fully developed in response to an initial brief application of the compound but instead developed progressively with repeated exposure to the propofol-containing test mixture. As shown by the Figure 8 data, supplementing the pipette solution with propofol (and, thus, presumably facilitating the access of propofol to the inner side of the plasma membrane) did not alter the potentiating effect of externally presented propofol. It is thus unlikely that the dependence of propofol's full action on repeated applications reflected a requirement for gradual passage of the compound across the cell membrane. Rather, the progressive nature of the effects of propofol appeared to reflect a cumulative action that built with repeated or prolonged exposure to the compound.
Among the effects of propofol exhibiting a dependence on repeated applications is an increase in the time constant of response deactivation after presentation of (G + T + P). Previous studies23,31,32 of hippocampal neurons have presented evidence for a lock-in of GABAA receptor-bound GABA by propofol (i.e., a propofol-dependent stabilization of the GABA-bound state). The present results (Fig. 5D) show that at high concentrations of propofol, the deactivation time constant of responses to serial (G + T + P) applications increases to values significantly exceeding those observed in the absence of propofol, consistent with the operation of a similar lock-in effect at bipolar cell GABAA receptors.
Although repeated brief applications of (G + T + P) at the mid-range propofol concentration of 150 μM led typically to a progressive increase in response potentiation (Figs. 4, 5), variations were observed in the waveform of the “stabilized” (G + T + P) response at a given propofol concentration and in the response elicited by (G + T + P) during propofol perfusion (Figs. 6A, 6B). That is, the stabilized (G + T + P) responses of some cells (e.g., Fig. 6A) at 150 μM propofol exhibited a waveshape representing propofol's more extensively developed action—a relatively rapid onset and a rebound exhibited on repeated treatment with propofol (e.g., purple trace in Fig. 4C). By contrast, the stabilized (G + T + P) response of other cells exhibited relatively slow onset and little, if any, sag or rebound component (e.g., black trace in Fig 6B). However, at propofol concentrations of 500–1500 μM, the investigated cells more regularly exhibited a waveshape characteristic of extensive propofol action (data not shown). The differential behavior observed at 150 μM propofol (Figs. 6A, 6B) might reflect, for example, a variability, among cells, in the concentration of propofol that produces a criterion extent of change in peak response, sag, and/or rebound in the response to a given experimental run within a series of applications. Consistent with this interpretation, there was relatively high variability in the extent of response sag (determinations of Ioff/Ipeak) and rebound [determinations of (Irebound − Ioff)/(Ipeak − Ioff)] at intermediate propofol concentrations and/or in later experimental runs of a series of test mixture applications (Figs. 5C, 5D).
Basis of Response Sag
Propofol at higher concentrations, in addition to causing a potentiation of the GABA-elicited response, induced a pronounced subsequent decrease from peak amplitude (i.e., development of a sag in the response). Furthermore, repeated applications of the propofol-containing test mixture progressively increased the magnitude of this sag and a rebound of current on the cessation of propofol application (Figs. 4, 5C). These results are consistent with the possibility that receptor desensitization in the presence of applied GABA contributes substantially to the observed sag. Desensitization by GABA is a well-established property of GABAA receptors, and, in the present study, the decrease in peak amplitude of the response to repeated (G + T + P) applications is more pronounced at 30 μM GABA than at 10 μM GABA (Figs. 7E, 7F). However, the present data further suggest a contribution to the sag from a receptor-blocking action of propofol. This possibility is raised by properties of the rebound component observed immediately on propofol cessation. That is, a simple possible basis of this rebound is the rapid dissociation of a blocker (propofol) from GABA-bound receptors that are already in the active state. Such a blocking activity of propofol is also consistent with the results obtained with propofol or (G + T) in the perfusing medium (Figs. 6A, 6C, respectively). In the former case, the evident elimination of the rebound component is consistent with the maintained presence of propofol during response deactivation; in the latter case, removal of the putative blocker (propofol) with maintained presence of GABA readily explains the observed increase in size of the rebound component. Previous studies have reported an inhibitory effect of high concentrations of propofol at GABAA receptors20,22 and have shown that etomidate, a compound whose binding site overlaps with (albeit is distinct from) the propofol binding site,33 can at high concentration exhibit channel-blocking activity.34–36
Dependence of Potentiation on Repeated Propofol Application
The above discussion of the Figures 4 and 5 data has pointed out the progressive increase in propofol's potentiating activity with repeated brief (2-second) applications of (G + T + P) despite relatively long (∼15- to 25-second) periods of Ringer perfusion between applications. The progressive nature of this increase indicates an effective memory of the potentiation process—that is, an effect of preceding (G + T + P) applications that lingers over the ∼15- to 25-second washout period. By contrast, Figures 6C and 6D indicate a recovery of the (G + T) response to the unpotentiated level on a subsecond time scale after propofol cessation, evidenced by the deactivation time constant of ∼400 ms. This contrast in the time scales of potentiation memory and return to the unpotentiated state together suggest the occurrence of a propofol-binding event that is inert (does not in itself generate the potentiated state) but that, on further presentation of propofol, directly or indirectly leads to receptor potentiation. One possibility consistent with this notion is that binding sites residing within the cell membrane but independent of the GABAA receptor compete with GABAA for applied propofol. On this possibility, the dependence of propofol's potentiating action on its repeated presentation (Figs. 4, 5, 7) reflects the “diversion” of initially added propofol by this high-affinity (but finite-capacity) independent binding process, and the potentiation observed with further propofol delivery corresponds to a condition of near-saturation of this competing process. The experimental data are also consistent with a second, quite different, possibility, that of a site on the GABAA receptor itself that, on occupation by propofol, produces a “primed” but unpotentiated state of the receptor. On this latter possibility, initial brief applications of (G + T + P) or initial exposures to propofol in the perfusing medium generate a propofol-bound, primed receptor state that exhibits a pronounced, rapidly developing potentiated response on subsequent exposure to (G + T + P) (Fig. 7). For example, if the propofol-sensitive GABAA receptors of bipolar cells contain two propofol binding sites, the primed (but unpotentiated) and potentiated receptor states might correspond respectively, with propofol binding at one versus both sites. That is, the potentiated receptor state could correspond with propofol binding at both a high-affinity “priming” site and a low-affinity “potentiating” site, and transition of the receptor from the potentiated to unpotentiated (but primed) state (Fig. 6C) could reflect propofol dissociation only from the low-affinity site.
Significance for ERG Analysis
In light of the central contribution of bipolar cell activity to the electroretinogram37–39 (see Ref. 40 for review), it is reasonable to hypothesize that propofol's potentiating action on bipolar cell GABAA receptors contributes to the observed effects of propofol on the b-wave of the full-field ERG and the p1 component of the multifocal ERG.24,25,41 However, propofol is known to exert multiple effects in neural tissue in addition to modulation of GABAA receptor activity.42–47 Among these additional sites of action are glycine receptors and cyclic nucleotide–gated HCN1 channels. Inasmuch as retinal neurons have receptors identical or similar to these additional target receptors/channels,42–50 propofol's action on the ERG may in substantial part reflect modulation of these and/or other “non-GABAA” mechanisms. Future studies testing the effect of propofol in combination with other agents that suppress specific ERG components51–53 may help to identify the possibly multiple mechanisms of action of propofol in the retina. Reciprocally, clarification of these mechanisms could enable the use of propofol as a pharmacologic tool for investigating signaling pathways within the retina.
Acknowledgments
The authors thank Robert F. Standaert for helpful discussions and Feng Feng for technical assistance during the course of this study.
Footnotes
Supported by National Institutes of Health Grants EY016094 and EY001792, the Daniel F. and Ada L. Rice Foundation (Skokie, IL), Hope for Vision (Washington, D.C.), the American Health Assistance Foundation (Clarksburg, MD), and Research to Prevent Blindness (New York, NY).
Disclosure: L. Yue, None; A. Xie, None; K.S. Bruzik, None; B. Frølund, None; H. Qian, None; D.R. Pepperberg, None
References
- 1. Feigenspan A, Wässle H, Bormann J. Pharmacology of GABA receptor Cl− channels in rat retinal bipolar cells. Nature. 1993;361:159–162 [DOI] [PubMed] [Google Scholar]
- 2. Qian H, Dowling JE. GABAA and GABAC receptors on hybrid bass retinal bipolar cells. J Neurophysiol. 1995;74:1920–1928 [DOI] [PubMed] [Google Scholar]
- 3. Palmer MJ. Functional segregation of synaptic GABAA and GABAC receptors in goldfish bipolar cell terminals. J Physiol. 2006;577:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lukasiewicz PD. Synaptic mechanisms that shape visual signaling at the inner retina. Prog Brain Res. 2005;147:205–218 [DOI] [PubMed] [Google Scholar]
- 5. Fletcher EL, Koulen P, Wässle H. GABAA and GABAC receptors on mammalian rod bipolar cells. J Comp Neurol. 1998;396:351–365 [DOI] [PubMed] [Google Scholar]
- 6. Euler T, Wässle H. Different contributions of GABAA and GABAC receptors to rod and cone bipolar cells in a rat retinal slice preparation. J Neurophysiol. 1998;79:1384–1395 [DOI] [PubMed] [Google Scholar]
- 7. Frech MJ, Backus KH. Characterization of inhibitory postsynaptic currents in rod bipolar cells of the mouse retina. Vis Neurosci. 2004;21:645–652 [DOI] [PubMed] [Google Scholar]
- 8. Greferath U, Müller F, Wässle H, Shivers B, Seeburg P. Localization of GABAA receptors in the rat retina. Vis Neurosci. 1993;10:551–561 [DOI] [PubMed] [Google Scholar]
- 9. Grigorenko EV, Yeh HH. Expression profiling of GABAA receptor β-subunits in the rat retina. Vis Neurosci. 1994;11:379–387 [DOI] [PubMed] [Google Scholar]
- 10. Enz R, Brandstätter JH, Hartveit E, Wässle H, Bormann J. Expression of GABA receptor ρ1 and ρ2 subunits in the retina and brain of the rat. Eur J Neurosci. 1995;7:1495–1501 [DOI] [PubMed] [Google Scholar]
- 11. Yeh HH, Grigorenko EV, Veruki ML. Correlation between a bicuculline-resistant response to GABA and GABAA receptor ρ1 subunit expression in single rat retinal bipolar cells. Vis Neurosci. 1996;13:283–292 [DOI] [PubMed] [Google Scholar]
- 12. Trapani G, Altomare C, Sanna E, Biggio G, Liso C. Propofol in anesthesia. Mechanism of action, structure-activity relationships, and drug delivery. Curr Med Chem. 2000;7:249–271 [DOI] [PubMed] [Google Scholar]
- 13. Olsen RW, Sieghart W. GABAA receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mascia MP, Trudell JR, Harris RA. Specific binding sites for alcohols and anesthetics on ligand-gated ion channels. Proc Natl Acad Sci U S A. 2000;97:9305–9310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krasowski MD, Hong X, Hopfinger AJ, Harrison NL. 4D-QSAR analysis of a set of propofol analogues: mapping binding sites for an anesthetic phenol on the GABAA receptor. J Med Chem. 2002;45:3210–3221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kitamura A, Sato R, Marszalec W, Yeh JZ, Ogawa R, Narahashi T. Halothane and propofol modulation of γ-aminobutyric acidA receptor single-channel currents. Anesth Analg. 2004;99:409–415 [DOI] [PubMed] [Google Scholar]
- 17. Franks NP. Molecular targets underlying general anaesthesia. Br J Pharmacol. 2006;147:S72–S81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Venkatachalan SP, Czajkowski C. A conserved salt bridge critical for GABAA receptor function and loop C dynamics. Proc Natl Acad Sci U S A. 2008;105:13604–13609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Olsen RW. The molecular mechanism of action of general anesthetics: structural aspects of interactions with GABAA receptors. Toxicol Lett. 1998;100–101:193–201 [DOI] [PubMed] [Google Scholar]
- 20. Orser BA, Wang L-Y, Pennefather PS, MacDonald JF. Propofol modulates activation and desensitization of GABAA receptors in cultured murine hippocampal neurons. J Neurosci. 1994;14:7747–7760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reynolds JN, Maitra R. Propofol and flurazepam act synergistically to potentiate GABAA receptor activation in human recombinant receptors. Eur J Pharmacol. 1996;314:151–156 [DOI] [PubMed] [Google Scholar]
- 22. Bali M, Akabas MH. Defining the propofol binding site location on the GABAA receptor. Mol Pharmacol. 2004;65:68–76 [DOI] [PubMed] [Google Scholar]
- 23. Bai D, Pennefather PS, MacDonald JF, Orser BA. The general anesthetic propofol slows deactivation and desensitization of GABAA receptors. J Neurosci. 1999;19:10635–10646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kommonen B, Hyvätti E, Dawson WW. Propofol modulates inner retina function in Beagles. Vet Ophthalmol. 2007;10:76–80 [DOI] [PubMed] [Google Scholar]
- 25. Ng Y-F, Chan HHL, To C-H, Yap MKH. The characteristics of multifocal electroretinogram in isolated perfused porcine eye: cellular contributions to the in vitro porcine mfERG. Doc Ophthalmol. 2008;117:205–214 [DOI] [PubMed] [Google Scholar]
- 26. Ramsey DJ, Ripps H, Qian H. Streptozotocin-induced diabetes modulates GABA receptor activity of rat retinal neurons. Exp Eye Res. 2007;85:413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qian H, Li L, Chappell RL, Ripps H. GABA receptors of bipolar cells from the skate retina: actions of zinc on GABA-mediated membrane currents. J Neurophysiol. 1997;78:2402–2412 [DOI] [PubMed] [Google Scholar]
- 28. Madsen C, Jensen AA, Liljefors T, et al. 5-Substituted imidazole-4-acetic acid analogues: synthesis, modeling, and pharmacological characterization of a series of novel γ-aminobutyric acidC receptor agonists. J Med Chem. 2007;50:4147–4161 [DOI] [PubMed] [Google Scholar]
- 29. Feigenspan A, Bormann J. GABA-gated Cl− channels in the rat retina. Prog Retin Eye Res. 1998;17:99–126 [DOI] [PubMed] [Google Scholar]
- 30. Vu TQ, Chowdhury S, Muni NJ, Qian H, Standaert RF, Pepperberg DR. Activation of membrane receptors by a neurotransmitter conjugate designed for surface attachment. Biomaterials. 2005;26:1895–1903 [DOI] [PubMed] [Google Scholar]
- 31. Williams DB, Akabas MH. Structural evidence that propofol stabilizes different GABAA receptor states at potentiating and activating concentrations. J Neurosci. 2002;22:7417–7424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chang Y, Weiss DS. Channel opening locks agonist onto the GABAC receptor. Nat Neurosci. 1999;2:219–225 [DOI] [PubMed] [Google Scholar]
- 33. Stewart D, Desai R, Cheng Q, Liu A, Forman SA. Tryptophan mutations at azi-etomidate photo-incorporation sites on α1 or β2 subunits enhance GABAA receptor gating and reduce etomidate modulation. Mol Pharmacol. 2008;74:1687–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hong Z, Wang D-S. Potentiation, activation and blockade of GABAA receptors by etomidate in the rat sacral dorsal commissural neurons. Neuroscience. 2005;132:1045–1053 [DOI] [PubMed] [Google Scholar]
- 35. Li G-D, Chiara DC, Sawyer GW, Husain SS, Olsen RW, Cohen JB. Identification of a GABAA receptor anesthetic binding site at subunit interfaces by photolabeling with an etomidate analog. J Neurosci. 2006;26:11599–11605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Campagna-Slater V, Weaver DF. Anaesthetic binding sites for etomidate and propofol on a GABAA receptor model. Neurosci Lett. 2007;418:28–33 [DOI] [PubMed] [Google Scholar]
- 37. Miller RF, Dowling JE. Intracellular responses of the Müller (glial) cells of mudpuppy retina: their relation to b-wave of the electroretinogram. J Neurophysiol. 1970;33:323–341 [DOI] [PubMed] [Google Scholar]
- 38. Xu X, Karwoski CJ. Current source density (CSD) analysis of retinal field potentials. I. Methodological considerations and depth profiles. J Neurophysiol. 1994;72:84–95 [DOI] [PubMed] [Google Scholar]
- 39. Tian N, Slaughter MM. Functional properties of a metabotropic glutamate receptor at dendritic synapses of ON bipolar cells in the amphibian retina. Vis Neurosci. 1995;12:755–765 [DOI] [PubMed] [Google Scholar]
- 40. Robson JG, Frishman LJ. Dissecting the dark-adapted electroretinogram. Doc Ophthalmol. 1999;95:187–215 [DOI] [PubMed] [Google Scholar]
- 41. Tanskanen P, Kylmä T, Kommonen B, Karhunen U. Propofol influences the electroretinogram to a lesser degree than thiopentone. Acta Anaesthesiol Scand. 1996;40:480–485 [DOI] [PubMed] [Google Scholar]
- 42. Chen X, Shu S, Bayliss DA. Suppression of Ih contributes to propofol-induced inhibition of mouse cortical pyramidal neurons. J Neurophysiol. 2005;94:3872–3883 [DOI] [PubMed] [Google Scholar]
- 43. Chen X, Shu S, Bayliss DA. HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine. J Neurosci. 2009;29:600–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krampfl K, Cordes A-L, Schlesinger F, Wolfes H, Bufler J. Effects of propofol on recombinant AMPA receptor channels. Eur J Pharmacol. 2005;511:1–7 [DOI] [PubMed] [Google Scholar]
- 45. Ying S-W, Abbas SY, Harrison NL, Goldstein PA. Propofol block of Ih contributes to the suppression of neuronal excitability and rhythmic burst firing in thalamocortical neurons. Eur J Neurosci. 2006;23:465–480 [DOI] [PubMed] [Google Scholar]
- 46. Lyashchenko AK, Redd KJ, Yang J, Tibbs GR. Propofol inhibits HCN1 pacemaker channels by selective association with the closed states of the membrane embedded channel core. J Physiol. 2007;583:37–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Haeseler G, Karst M, Foadi N, et al. High-affinity blockade of voltage-operated skeletal muscle and neuronal sodium channels by halogenated propofol analogues. Br J Pharmacol. 2008;155:265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dong X-P, Xu T-L. The actions of propofol on γ-aminobutyric acidA and glycine receptors in acutely dissociated spinal dorsal horn neurons of the rat. Anesth Analg. 2002;95:907–914 [DOI] [PubMed] [Google Scholar]
- 49. Higuchi H, Funahashi M, Miyawaki T, et al. Suppression of the hyperpolarization-activated inward current contributes to the inhibitory actions of propofol on rat CA1 and CA3 pyramidal neurons. Neurosci Res. 2003;45:459–472 [DOI] [PubMed] [Google Scholar]
- 50. Cacheaux LP, Topf N, Tibbs GR, et al. Impairment of hyperpolarization-activated, cyclic nucleotide-gated channel function by the intravenous general anesthetic propofol. J Pharmacol Exp Ther. 2005;315:517–525 [DOI] [PubMed] [Google Scholar]
- 51. Bush RA, Sieving PA. A proximal retinal component in the primate photopic ERG a-wave. Invest Ophthalmol Vis Sci. 1994;35:635–645 [PubMed] [Google Scholar]
- 52. Robson JG, Frishman LJ. Response linearity and kinetics of the cat retina: the bipolar cell component of the dark-adapted electroretinogram. Vis Neurosci. 1995;12:837–850 [DOI] [PubMed] [Google Scholar]
- 53. Dong C-J, Hare WA. Contribution to the kinetics and amplitude of the electroretinogram b-wave by third-order retinal neurons in the rabbit retina. Vision Res. 2000;40:579–589 [DOI] [PubMed] [Google Scholar]