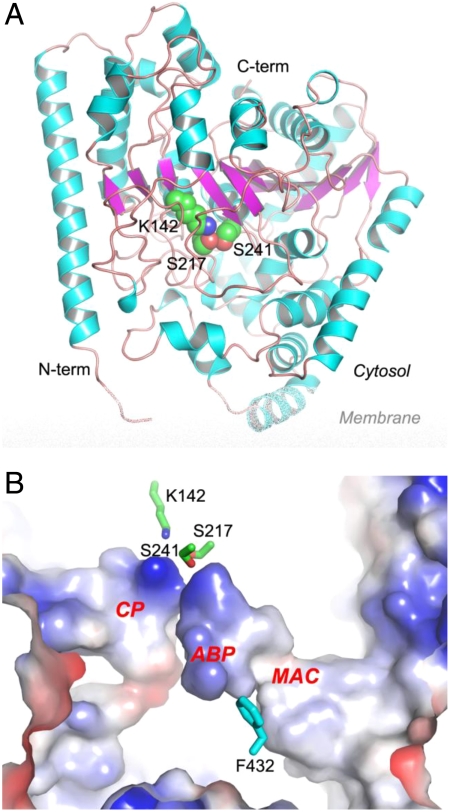
Fig. 2.
Crystal structure of apo rat FAAH. (A) Overall structure of FAAH monomer in ribbon representation with the center β-sheet colored in magenta, surrounding α-helices in cyan, and loops in pink. The catalytic triad of Ser241-Ser217-Lys142 is shown in spheres with atomic color of carbon in green, oxygen in red, and nitrogen in blue. (B) Active site of FAAH in electrostatic surface representation. Three major cavities in the active site are highlighted as the membrane access channel (MAC), the acyl-chain binding pocket (ABP), and the cytosolic port (CP). The catalytic triad and residue Phe432 are shown in sticks.