A central question in epidemiology is the extent to which vaccines provide indirect protection (herd immunity) to infectious diseases, in addition to directly protecting individuals. Such artificial immunity often matches with the strength of natural protection: Vaccines against infections with strong and long-lasting natural immunity, such as measles, often provide very durable and effective protection against both disease and transmission (1, 2). A more complex picture emerges with pertussis (whooping cough), which is caused by a bacterium, Bordetella pertussis. The disease burden arising from this serious childhood infection is still considerable in countries with low vaccination rates. On the other hand, over the last 60 y, there has been a major reduction in pertussis in many countries, associated with mass vaccination of infants and toddlers (3). However, in the last decade or so, infection has resurged in many highly vaccinated populations (4). Further, the age distribution of pertussis has changed markedly: Disease (and likely infection) used to be focused in the very young, as in a classical immunizing infection; in the recent resurgence, clinical disease has a much wider age range, with a conspicuous peak in adolescents. A number of recent studies have focused on the question of pertussis immunity and resurgence. The paper by Lavine et al. in PNAS (5) presents a distinctive explanation for these phenomena on the basis of temporal changes in boosting of prevailing immunity by exposure to pertussis infection in the community.
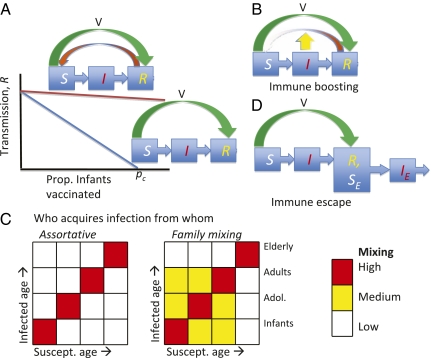
The impact of vaccination on transmission is neatly expressed via the effective reproduction ratio of infection (R) (1, 2). This parameter is the number of secondary cases that an infected individual would generate at a given level of population susceptibility. For infections engendering strong and long-lasting protection following natural or artifical immunization, R declines with the level of vaccination (Fig. 1A), eventually reaching a herd immunity threshold (R < 1) where the infection cannot reinvade the population; this pattern corresponds to the classic susceptible–infected–recovered (SIR) paradigm for epidemic dynamics (1). By contrast, if there is significant loss of immunity, as embodied in the susceptible–infected–recovered–susceptible (SIRS) paradigm (6), R declines hardly at all with vaccination (Fig. 1A) and there is relatively little herd immunity. As well as the applied implications for control strategies, the extent of scaling of transmission with vaccination can profoundly affect the cyclical nature of epidemics, by tuning the strength of nonlinear feedback between susceptible and infected numbers.
Fig. 1.
Factors influencing the dynamics of pertussis infection. (A) Scaling of transmission with vaccination rate for strongly immunizing (SIR) infections and infections with significant loss of immunity (SIRS). (B) Immune boosting, by exposure to currently circulating infection, could inhibit immunity loss (and hence the flow from R back to S) (5). (C) Patterns of population mixing and infection. Assortative mixing by age (Left matrix) could lessen the transmission impact of immune loss in older individuals, compared with more widespread age mixing (Right matrix) (13). (D) Novel genetic variants could escape immunity (12). In this cartoon, individuals immune to the prevailing pathogen genotype (in class R) are susceptible to an invading genotype (hence, they are also in class SE and can become infected with the genotype, moving into IE.
The recent rise in pertussis cases appears to be more than simply an increase in the efficiency of surveillance (5). Lavine et al.’s paper joins a long history of pertussis epidemic modeling (6–10), as well as a body of recent work that explores explanations for the recent rise in adolescent cases (11–13). In terms of overall pertussis dynamics, comparative epidemiological studies across countries extract a strong signal of natural and vaccinal herd immunity in historical pertussis dynamics (11). Lavine et al. (5) aim to reconcile this evidence for historical herd immunity, in comparison with recent outbreaks in highly vaccinated populations. They achieve this with epidemiological models, rooted in detailed surveillance data from Massachusetts (4). Previous models have considered the impact of boosting by subclinical infection on the strength of immunity against pertussis and other infections (e.g., refs. 10 and 14). The rationale here is that, before mass vaccination, previously acquired immunity against a given pathogen is boosted by subclinical exposure to the infection circulating in the population. By contrast, when incidence of infection falls in the vaccine era, average reexposure to pathogen antigens is reduced. This reduction in turn could then reduce the duration of natural and vaccinal immunity at the individual level and hence herd protection for the population (Fig. 1B).
Lavine et al.’s approach to immune boosting is distinctive because they focus in particular on the dose of pertussis antigen necessary to achieve immune boosting. In contrast to previous work, they hypothesize that boosting of previously acquired immunity could take a smaller dose of antigen than required to cause a primary immune response; this assumption accords with experimental evidence on the dynamics of primed T and B cells. Lavine et al. test this hypothesis by fitting an age-structured epidemiological model to a detailed database on age-structured clinical cases of pertussis in Massachusetts from 1998 to 2008. They use an elegantly simple age-structured model; a tunable degree of boosting allows them to test their dosage hypothesis. Results of the fitting show that they can best capture the observed increases in pertussis incidence and age at infection if immune boosting is more easily triggered than primary infection and immunity. This finding corroborates their boosting hypothesis and allows them to explore the hitherto unexplained shift in herd immunity that pertussis seems to have shown as vaccination increased. Their model indicates that pertussis immunity in the prevaccination era was sufficiently strongly boosted by circulation of infection to drive essentially SIR-type dynamics. By contrast, in highly vaccinated populations, boosting is sufficiently weakened to move the system much more toward a cyclical variant of SIRS dynamics.
Lavine et al. explore the dynamics of these models with a lucid combination of simulation and dynamical systems approaches. Because they deliberately choose parsimonious models, their paper (as they say themselves) averages across many details of the biology, to be explored in future work. One important area for development is the impact of heterogeneity in transmission with host age (Lavine et al. assume homogeneous mixing). Such heterogeneities are generally incorporated in epidemiological models via an age-specific matrix recording “who acquires infection from whom” (WAIFW) by age (1) (Fig. 1C). The WAIFW matrix can be parameterized by diary-based surveys of linkage (15); these contacts often peak within young childhood cohorts, with contact patterns across age classes that reflect family, school, work, etc., networks. A recent study of pertussis dynamics in Sweden (13) shows that heterogeneities in age-specific contact patterns may be particularly important when immunity to infection is imperfect. In particular, highly age-assortative mixing (Fig. 1C, Left) may limit the impact of loss of immunity in older, less well mixed, age classes on infection in younger people, compared with less restricted patterns, based for example on mixing within families (Fig. 1C, Right). Essentially, this focusing of infection pushes the system toward SIR-type dynamics (13). An important area for development is to extend Lavine et al.’s boosting models to explore the dynamic impact of such age-specific heterogeneities in transmission, as well as more general spatial and seasonal heterogeneities in mixing. This exercise would illuminate both the causes of historical pertussis dynamics and the impact of currently proposed control strategies, such as adolescent vaccination and “cocooning” of infants by adult vaccination (3). A further explanation for the recent upsurge of pertussis is rooted in the genetics and evolutionary dynamics of the pathogen (12, 16). Specifically, if vaccination has selected for new variants (for example, in respect to the key pertussis toxin of the bacterium), which are able to invade current vaccinal immunity, this mechanism could contribute to the recent increase in cases. Recent work on variation in pertussis toxin in Holland (12) proposes that reduction in primary infant susceptible numbers by vaccination programs may have selected pertussis toxin variants that could preferentially infect immunized individuals. This focus on the dynamics of repeat infections opens interesting parallels with the boosting story; models integrating boosting into phylodynamic models, which synthesize pertussis evolution and epidemiology (16, 17), are again an important area for future work.
However, to achieve any synthesis of explanations for pertussis dynamics, the key need is for further datasets to help distinguish how the combination of host mixing, immunodynamics, and pathogen strain structure drives the impact of vaccination. First, a broader range of comparative epidemiological studies is needed, particularly extending the collection of age-structured surveillance data and pathogen genotyping to contemporary settings where pertussis vaccination is not widespread (12). Second, explicit quantification of the linkage between population mixing and patterns of transmission is needed. For instance, it would be instructive to extend important studies recording spread of infection from adults to infants within families (e.g., ref. 18) to quantify resulting patterns of transmission and herd immunity in the broader population. Third, Lavine et al. (5) crystallize a testable hypothesis about the relative dose of antigen necessary to boost existing immunity, compared with eliciting primary immunity. It would be instructive to examine this prediction, for example via animal transmission models, both in terms of pertussis and for its impact on the dynamics of other infections.
Pertussis continues to exert a major burden of disease worldwide, with significant morbidity even in highly vaccinated populations. It also represents an important case study on the impact of biological and social drivers of epidemic dynamics in imperfectly immunizing infections. The paper by Lavine et al. (5) proposes an ingenious explanation for observed transitions in pertussis dynamics, in response to vaccination; more generally, it also explores important territory in the link between individual- and population-level manifestations of immunity.
Footnotes
The author declares no conflict of interest.
See companion article on page 7259 in issue 17 of volume 108.
References
- 1.Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. Oxford: Oxford Univ Press; 1992. [Google Scholar]
- 2.Keeling MJ, Rohani P. Modeling Infectious Diseases in Humans and Animals. Princeton: Princeton Univ Press; 2007. [Google Scholar]
- 3.Forsyth KD, et al. Global Pertussis Initiative New pertussis vaccination strategies beyond infancy: Recommendations by the global pertussis initiative. Clin Infect Dis. 2004;39:1802–1809. doi: 10.1086/426020. [DOI] [PubMed] [Google Scholar]
- 4.Lavine J, Broutin H, Harvill ET, Bjørnstad ON. Imperfect vaccine-induced immunity and whooping cough transmission to infants. Vaccine. 2010;29:11–16. doi: 10.1016/j.vaccine.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavine JS, King AA, Bjornstad ON. Natural immune boosting in pertussis dynamics and the potential for long-term vaccine failure. Proc Natl Acad Sci USA. 2011;108:7259–7264. doi: 10.1073/pnas.1014394108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hethcote HW. An age-structured model for pertussis transmission. Math Biosci. 1997;145:89–136. doi: 10.1016/s0025-5564(97)00014-x. [DOI] [PubMed] [Google Scholar]
- 7.Fine PEM, Clarkson JA. Distribution of immunity to pertussis in the population of England and Wales. J Hyg (Lond) 1984;92:21–36. doi: 10.1017/s0022172400063993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fine PEM, Clarkson JA. Reflections on the efficacy of pertussis vaccines. Rev Infect Dis. 1987;9:866–883. doi: 10.1093/clinids/9.5.866. [DOI] [PubMed] [Google Scholar]
- 9.Rohani P, Earn DJD, Grenfell BT. Opposite patterns of synchrony in sympatric disease metapopulations. Science. 1999;286:968–971. doi: 10.1126/science.286.5441.968. [DOI] [PubMed] [Google Scholar]
- 10.Wearing HJ, Rohani P. Estimating the duration of pertussis immunity using epidemiological signatures. PLoS Pathog. 2009;5:e1000647. doi: 10.1371/journal.ppat.1000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broutin H, Viboud C, Grenfell BT, Miller MA, Rohani P. Impact of vaccination and birth rate on the epidemiology of pertussis: A comparative study in 64 countries. Proc R Soc B Biol Sci. 2010;277:3239–3245. doi: 10.1098/rspb.2010.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mooi FR. Bordetella pertussis and vaccination: The persistence of a genetically monomorphic pathogen. Infect Genet Evol. 2010;10:36–49. doi: 10.1016/j.meegid.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Rohani P, Zhong X, King AA. Contact network structure explains the changing epidemiology of pertussis. Science. 2010;330:982–985. doi: 10.1126/science.1194134. [DOI] [PubMed] [Google Scholar]
- 14.Garnett GP, Grenfell BT. The epidemiology of varicella-zoster virus infections: The influence of varicella on the prevalence of herpes zoster. Epidemiol Infect. 1992;108:513–528. doi: 10.1017/s0950268800050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mossong J, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. Plos Med. 2008;5:381–391. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Boven M, Mooi FR, Schellekens JF, de Melker HE, Kretzschmar M. Pathogen adaptation under imperfect vaccination: Implications for pertussis. Proc R Soc B Biol Sci. 2005;272:1617–1624. doi: 10.1098/rspb.2005.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Restif O, Grenfell BT. Vaccination and the dynamics of immune evasion. J R Soc Interface. 2007;4:143–153. doi: 10.1098/rsif.2006.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Greeff SC, et al. Pertussis disease burden in the household: How to protect young infants. Clin Infect Dis. 2010;50:1339–1345. doi: 10.1086/652281. [DOI] [PubMed] [Google Scholar]