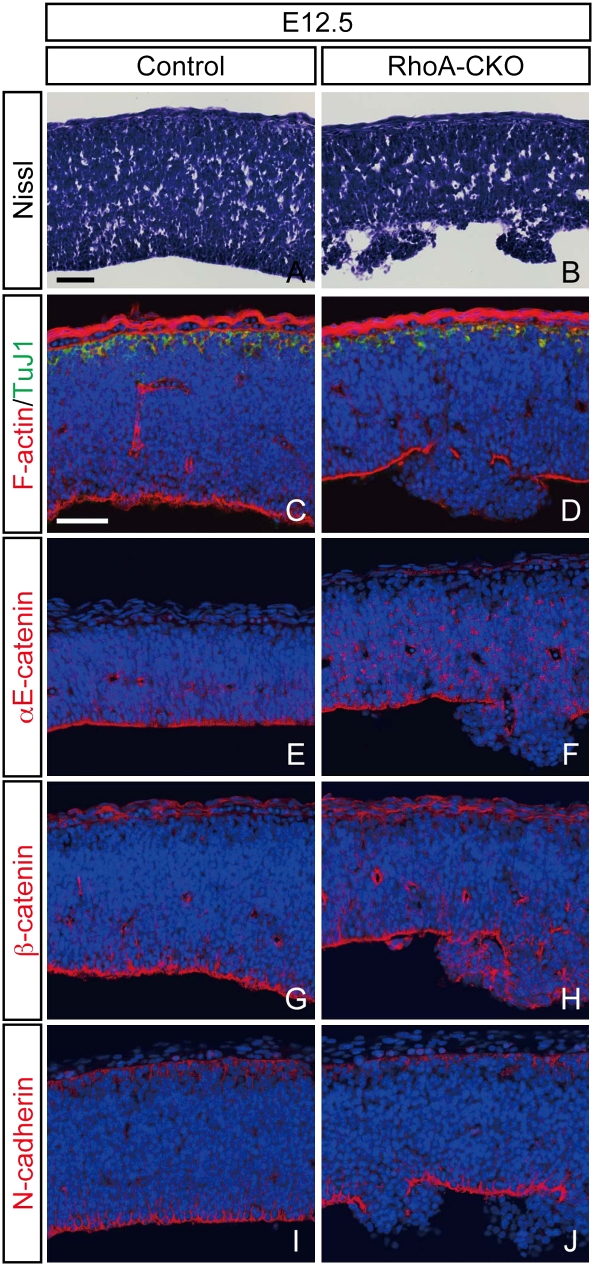
Fig. 3.
Disruption of adherens junctions in RhoA-deleted neural progenitor cells. (A and B) Nissl-stained sections through the developing mesencephalon from control (A) and RhoA-CKO (B) embryos at E12.5. The apical structure is partially disrupted in RhoA-CKO embryos, and neural progenitor cells are irregularly arranged and protruded to the ventricle in this region (B). (C and D) Double immunostaining for F-actin and βIII-tubulin of control (C) and RhoA-CKO (D) embryos. Apical F-actin staining is disrupted in the dysplastic region, whereas basal βIII-tubulin (TuJ1) staining remains unaffected (D). (E–I) Immunostaining for αE-catenin (E and F), β-catenin (G and H), and N-cadherin (I and J) of control (E, G, and I) and RhoA-CKO (F, H, and J) embryos at E12.5. Apical staining of these molecules is also disrupted in the dysplastic region. (Scale bars, 50 μm.)