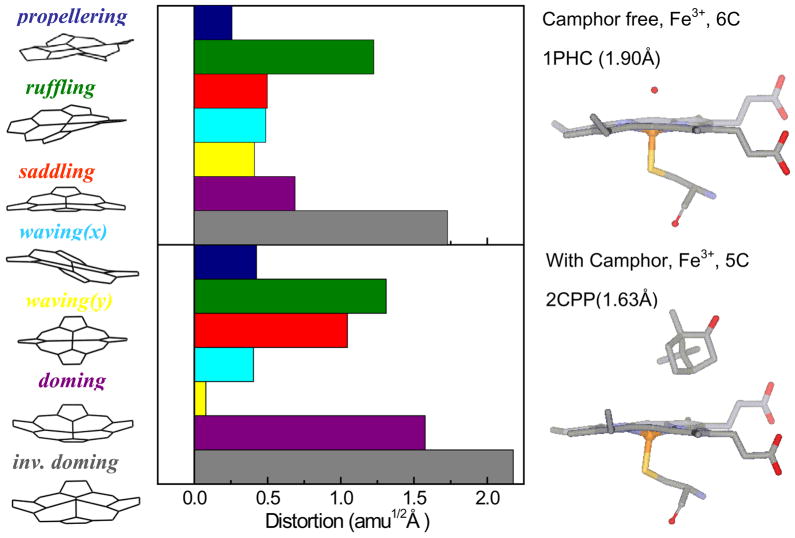
Figure 10.
Crystal structures and NSD analysis of the camphor-free and camphor-bound forms of the ferric heme of cytochrome P450cam. The displacement along each of the low frequency normal mode unit vectors of Fe porphine is given in mass weighted coordinates (amu1/2 Å). The color coding for the modes is pro: propellering (blue), ruf: ruffling (green), sad: saddling (red), wav(x): wavingx (light blue), wav(y): wavingy (yellow), dom: doming (purple), invdom: inverse doming (gray). The crystal structures are extracted from the protein data bank: 1PHC79 for the camphor-free and 2CPP72 for the camphor-bound form. One of the major differences is that the camphor-bound form has a strong heme doming distortion, which is consistent with the appearance of the ~33 cm−1 mode in the coherence spectra of camphor-bound P450cam (Fig. 3)