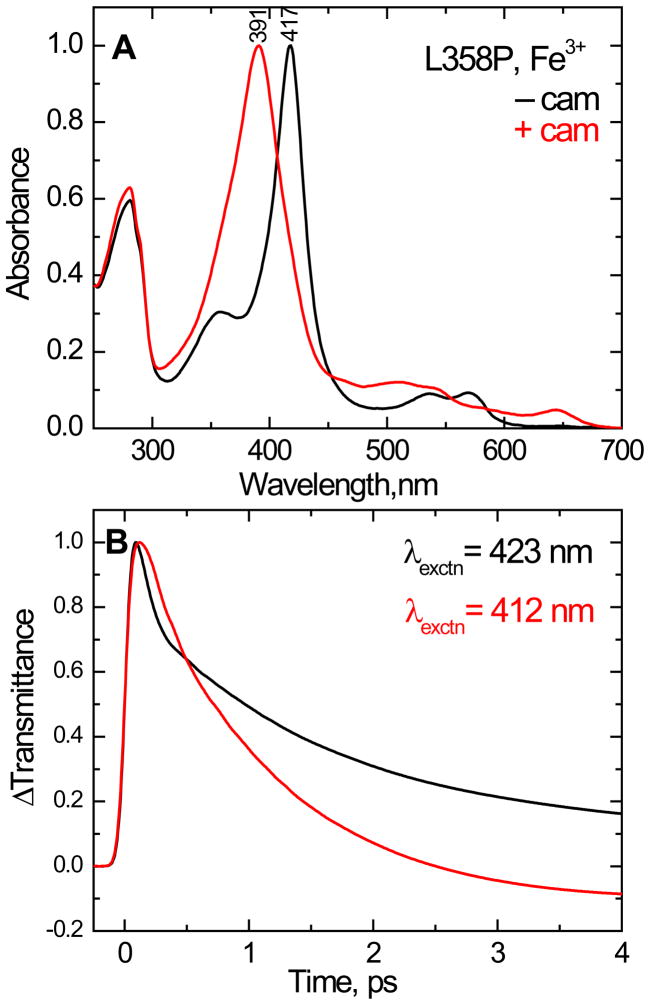
Figure 5.
(A) Normalized equilibrium electronic spectra of the camphor-free and camphor-bound ferric state of L358P. The Soret absorption maxima are 417 and 391 nm, respectively. (B) Normalized time resolved transmittance (ΔT) of the above complexes obtained with pump/probe excitation at 423 and 412 nm respectively. The kinetic traces show a bleaching signal (ΔT > 0) that recovers to equilibrium with kinetics that are similar to the wild type. The fitting parameters are for camphor free species τ1= 62 fs (a1 = 0.75), τ2= 1.5 ps (a2 = 0.21) and offset = 0.04 and for camphor bound complex τ1= 118 fs (a1 = 0.33), τ2= 1.1 ps (a2 = 0.74) and offset = − 0.07. The shortest time constant and amplitude are distorted due to the convolution with coherence coupling signal.