Abstract
Objective
To estimate the contribution of clinically-confirmed diabetes mellitus to tuberculosis (TB) rates in communities where both diseases are prevalent as a way to identify opportunities for TB prevention among diabetic patients.
Methods
This is a prospective study in which TB patients ≥ 20 years old at TB clinics in the Texas–Mexico border were tested for diabetes. The risk of tuberculosis attributable to diabetes was estimated from statistics for the corresponding adult population.
Findings
The prevalence of diabetes among TB patients was 39% in Texas and 36% in Mexico. Diabetes contributed 25% of the TB cases studied, whereas human immunodeficiency virus (HIV) infection contributed 5% or fewer. Among TB patients, fewer Mexicans than Texans were aware that they had diabetes before this study (4% and 19%, respectively). Men were also less frequently aware than women that they had diabetes (P = 0.03). Patients who knew that they had diabetes before the study had an 8-year history of the disease, on average, before being diagnosed with TB.
Conclusion
Patients with diabetes are at higher risk of contracting TB than non-diabetic patients. Integrating TB and diabetes control programmes worldwide would facilitate TB prevention among diabetes patients and increase the number of diabetics who learn of their condition, particularly among males. Such a strategy would lead to earlier case detection and improve the management of both TB and diabetes.
ملخص
الغرض تقدير إسهام حالات داء السكري الإكلينيكية في معدلات السل في المجتمعات التي ينتشر فيها كلا المرضين كوسيلة للتعرّف على فرص اتقاء السل بين مرضى السكري.
الطريقةفي هذه الدراسة الاستباقية جري اختبار مرضى السل البالغ عمرهم 20 سنة أو أكبر، وكانوا يراجعون العيادات الواقعة في المنطقة الحدودية بين تكساس والمكسيك لاختبار السكري. وجرى تقدير اختطار السل الذي يعزى إلى السكري من الإحصائيات المتناظرة للسكان البالغين في البلدين.
النتائج بلغ انتشار السكري بين مرضى السل 39% في تكساس و 36% في المكسيك. أسهم السكري في 25% من حالات السل التي جرى دراستها، بينما أسهمت عدوى فيروس العوز المناعي البشري في 5% أو أقل من ذلك. كان بين مرضى السل هناك عدد أقل من المكسيكيين على علم بإصابتهم بالسكري مقارنة بأهالي تكساس وذلك قبل إجراء الدراسة (4% مقابل 19% بالترتيب). وكان الرجال أيضاً أقل وعياً من النساء بإصابتهم بالسكري (قوة الاحتمال P= 0.03). المرضى الذين يعرفون إصابتهم بالسكري قبل الدراسة كان تاريخ إصابتهم بالسكري يصل إلى 8 سنوات في المتوسط قبل تشخيص إصابتهم بالسل.
الاستنتاج يتعرض مرضى السكري لاختطار الإصابة بالسل أعلى من غير المصابين بالسكري. ومن شأن الدمج بين برامج مكافحة السل والسكري على الصعيد العالمي أن يؤدي إلى تيسير الوقاية من السل بين مرضى السكري وزيادة عدد المصابين بالسكري الذين يعرفون أنهم مصابون بالمرض، ولاسيما بين الذكور. هذه الاستراتيجية ستؤدي إلى اكتشاف مبكر للحالات وتحسين معالجة كلا من السل والسكري.
摘要
目的
旨在评估糖尿病和结核病高发社区中临床确认的糖尿病对结核病(TB)的作用。
方法
这是一项前瞻性研究,本研究对德克萨斯-墨西哥边境结核病诊所的20岁及以上结核病人进行糖尿病测试。然后通过统计数字估测相应成人人群中归因为糖尿病的结核病风险。
结果
德克萨斯州和墨西哥结核病人中糖尿病的发病率分别为39%和36%。在所研究的病例中,糖尿病引发了25%的结核病例,而人体免疫缺损病毒(HIV)感染仅引起了5%或更少的结核病例。结核病人中,在本研究之前意识到自己患有糖尿病的墨西哥人比德克萨斯人要少(分别为4%和19%)。与女性相比,男性也较少意识到自己患有糖尿病(P=0.03)。在本研究之前得知自己患有糖尿病的患者在诊断出患有结核病之前平均已有8年的糖尿病史。
结论
糖尿病患者比非糖尿病患者感染结核病的风险要高。整合世界范围内,结核病和糖尿病控制方案将便于糖尿病患者的结核病预防并增加得知自己情况的糖尿病患者数量,特别是男性。该策略将促进较早的病例检测并改善结核病和糖尿病管理。
Резюме
Цель
Оценить влияние клинически подтвержденных случаев заболевания сахарным диабетом на показатели заболеваемости туберкулезом (ТБ) в целях выявления возможностей для профилактики ТБ среди больных диабетом.
Методы
Данная работа представляет собой проспективное исследование, в ходе которого все больные ТБ в возрасте от 20 лет и старше в туберкулезных клиниках на мексикано-американской (штат Техас) границе проходили тестирование на диабет. Риск развития туберкулеза, обусловленный диабетом, оценивался путем анализа статистики корреспондирующего взрослого населения.
Результаты
Распространенность диабета среди больных ТБ составляла 39% в Техасе и 36% iв Мексике. Диабет способствовал развитию туберкулеза в 25% изученных случаев заболевания ТБ, в то время как вирус иммунодефицита человека (ВИЧ) – в 5% случаев или менее. Среди больных ТБ, которые до проведения исследования знали, что больны диабетом, мексиканцев было меньше, чем техасцев (4 и 19%, соответственно), а мужчин меньше, чем женщин (P = 0.03). Больные, которые до проведения исследования знали, что больны диабетом, наблюдались у врача по поводу диабета в течение восьми лет, прежде чем у них был диагностирован ТБ.
Вывод
У диабетиков риск заболевания ТБ выше, чем у тех, кто не болен диабетом. Интеграция программ лечения ТБ и диабета в странах мира облегчила бы профилактику ТБ среди больных диабетом и способствовала бы увеличению численности диабетиков, знающих о том, что они больны, особенно среди мужчин. Такая стратегия привела бы к более раннему выявлению случаев заболевания и улучшила бы лечение как ТБ, так и диабета.
Resumen
Objetivo
Evaluar la contribución de la diabetes mellitus confirmada clínicamente a las tasas de tuberculosis (TB) en comunidades en las que ambas enfermedades son prevalentes, de manera que se puedan identificar las posibilidades de prevención de la TB entre los pacientes diabéticos.
Métodos
En este estudio prospectivo se realizaron diversas pruebas de detección de la diabetes, en pacientes con TB, con una edad igual o superior a 20 años, procedentes de clínicas para la tuberculosis situadas en la frontera entre Texas y México. El riesgo de tuberculosis atribuible a la diabetes se calculó a partir de las estadísticas para la población adulta correspondiente.
Resultados
La prevalencia de la diabetes entre los pacientes con TB fue del 39% en Texas y del 36% en México. La diabetes contribuyó en un 25% de los casos de TB estudiados, mientras que la infección por el virus de la inmunodeficiencia humana (VIH) contribuyó en un 5% o menos. Antes de este estudio, una cantidad menor de mexicanos que de texanos con tuberculosis desconocía padecer diabetes (4% y 19% respectivamente). La proporción de hombres que sabían que padecían diabetes fue menor que la de mujeres (p = 0,03). Los pacientes que sabían que padecían diabetes antes del estudio contaban con antecedentes médicos de la enfermedad, durante 8 años de media, antes de ser diagnosticados de TB.
Conclusión
Los pacientes con diabetes tienen un mayor riesgo de contraer tuberculosis que los pacientes que no la padecen. Los programas que integren el control de la diabetes y la tuberculosis en todo el mundo facilitarían la prevención de la tuberculosis entre los pacientes con diabetes y aumentarían el número de diabéticos que serían conocedores de su afección, especialmente entre los hombres. Una estrategia como ésta conllevaría una detección más precoz y mejoraría la gestión tanto de la tuberculosis como de la diabetes.
Résumé
Objectif
Évaluer la contribution aux taux de tuberculose (TB) du diabète sucré cliniquement avéré dans les communautés où les deux maladies sont répandues afin de l’utiliser comme méthode d’identification des possibilités de prévention de la TB chez les patients diabétiques.
Méthodes
Il s’agit d’une étude prospective au cours de laquelle des patient âgés de 20 ans et plus, atteints de tuberculose et hospitalisés dans des cliniques spécialisées de la frontière entre le Texas et le Mexique, ont subi des tests pour le diabète. Le risque de tuberculose attribuable au diabète a été évalué à partir des statistiques concernant la population adulte correspondante.
Résultats
La prévalence du diabète chez les patients atteints de tuberculose s’élevait à 39% au Texas et à 36% au Mexique. Le diabète représentait 25% des cas de TB étudiés, tandis que les infections provoquées par le virus de l’immunodéficience humaine (VIH) ne représentaient que 5% ou moins. Parmi les patients atteints de tuberculose, les Mexicains étaient moins nombreux que les Texans à savoir qu’ils étaient diabétiques avant cette étude (4% contre 19% respectivement). De même, les hommes étaient plus rarement informés de leur diabète que les femmes (P = 0,03). Les patients se sachant diabétiques avant cette étude étaient, en moyenne, malades depuis 8 ans avant que la TB ne soit diagnostiquée chez eux.
Conclusion
Les patients atteints de diabète risquent davantage de contracter la TB que les non-diabétiques. L’intégration à l’échelle planétaire des programmes de contrôle de la TB et du diabète faciliterait la prévention contre la TB chez les patients diabétiques et augmenterait le nombre de diabétiques informés de leur maladie, notamment chez les hommes. Ce type de stratégie permettrait de détecter plus tôt la maladie et d’améliorer la gestion tant de la TB que du diabète.
Introduction
Tuberculosis (TB) continues to be the leading killer among bacterial diseases worldwide. In 2009, more than 9 million new cases were diagnosed and 1.7 million people died from the disease.1 The World Health Organization (WHO) suspects that TB control is being undermined by the growing number of patients with diabetes mellitus in the world, which currently stands at an estimated 285 million but is anticipated to reach 438 million by 2030.2,3 Prior to the 1950s reports of an association between diabetes (primarily type 1) and TB were frequent in the literature, but they waned as insulin and drugs against TB became available.4,5 This association (now with type 2 diabetes) was recognized again in the 1990s6–9 and is currently supported by a growing body of literature.6–17 According to a recent meta-analysis, diabetes patients have three times the risk of contracting TB as non-diabetics (95% confidence interval, CI: 2.3–4.3)18 and studies report the fraction of TB cases attributable to diabetes to be between 15% and 25%.9,13,16 The biological basis for the association between both diseases is not fully understood but studies suggest that diabetes depresses the immune response, which in turn facilitates infection with Mycobacterium tuberculosis and/or progression to symptomatic disease. This is corroborated by the fact that diabetes is generally diagnosed before TB develops.4,19–22
Despite the suggested importance of diabetes as a risk factor for TB, most contemporary studies are based on secondary data or self-reported diabetes status.6–17 The contribution of diabetes to the burden of TB may be more conspicuous in countries where both diseases are highly prevalent: Bangladesh, Brazil, China, India, Indonesia, Pakistan, and the Russian Federation are high-burden countries and rank among the 10 countries with the highest numbers of diabetes patients2,23 and also classified as high-burden for TB. However, the risk of TB attributable to diabetes has only been reported for India and it was estimated from secondary data.13 We need to gain a deeper understanding of the differences between TB patients with and without diabetes to assist in the development of guidelines to prevent co-morbidity.
Our research team is strategically located on the Texas–Mexico border, where TB is endemic. In 2007 the overall incidence of TB was 10.5 cases per 100 000 in south Texas and 38 per 100 000 in north-eastern Mexico (personal communications, JL Robles, Secretaría de Salud de Tamaulipas [SSA-Tamaulipas]; Brian Smith and Nita Ngo, Texas Department of State and Health Services [DSHS]). Diabetes prevalence among adults over the age of 20 years is 19.4% in South Texas and 15.1% in north-eastern Mexico.24,25 Our previous studies were conducted with data extracted from existing surveillance databases for TB control and were based on self-reported diabetes.17 In this study our objective was to estimate the risk of TB attributable to diabetes in this population along the Mexican border using primary data from patients newly diagnosed with TB and tested for diabetes.
Methods
Patient enrolment
Referral patients with probable TB were enrolled between March 2006 and September 2008 at the TB clinics in Hidalgo and Cameron County Health Departments, which are the TB reference centres for South Texas counties (South Texas), and the SSA-Tamaulipas in Matamoros, Mexico (north-eastern Mexico). Jail inmates and individuals < 20 years of age were excluded. Individuals fulfilling the inclusion criteria but refusing to participate (n = 80; 95% from South Texas) did not differ with respect to age, gender, race or ethnicity from those enrolled (data not shown). Participants signed informed consent. The study was approved by the institutional review boards of the participating institutions in Mexico and the United States.
For TB diagnosis we used standard WHO definitions: culture positive for Mycobacterium tuberculosis (confirmed case), sputum smear positive for acid-fast bacilli when culture data were not available (smear-positive case), or clinical diagnosis only when microbiological test results were negative or not available (clinical case: symptoms compatible with TB and documentation of anti-TB treatment for at least 6 months).26 Participants in whom TB was ruled out or whose test results were inconclusive were excluded (Fig. 1). We used the American Diabetes Association classification guidelines for epidemiological studies: hyperglycaemia and/or self-reported diabetes.27 To measure blood glucose we used blood anticoagulated with ethylenediaminetetraacetic acid (EDTA) and a hand-held glucometer (AccuCheck Advantage, Roche Indianapolis, United States of America). We defined hyperglycaemia as fasting blood glucose ≥ 126 mg/dl or a random blood glucose ≥ 200 mg/dl. We froze whole EDTA-treated blood and batch tested it for glycosylated haemoglobin (HbA1c) (GLYCO-Tek Affinity Column, Beaumont, USA) and we defined chronic hyperglycaemia as HbA1c ≥ 6.5%.28 HIV antibody tests were performed on 222 (95%) of the TB patients at the health departments or in our laboratory (Mulitspot HIV-1/HIV-2 rapid test; Biorad Laboratories, Redmond, USA). In all other cases (11, or 5%) HIV status was based on self-reporting. Using the validated quantity frequency questions described previously, we defined alcohol abuse on the basis of consumption frequency and on the average number of alcoholic drinks consumed in a typical day.29 We defined alcohol abuse as at least one weekly episode of drinking seven drinks or more or binge drinking (at least two monthly episodes of drinking 10 drinks or more). Past or present drug abuse was self-reported. Household income was self-reported and we have reported the data in dollars (average exchange rate of 11 Mexican pesos to one United States dollar (US$) during the study period). We recorded height and weight to calculate the body mass index [BMI, (weight in pounds × 703)/(height in inches)2] and we classified patients as underweight (BMI < 18·5), normal (18·5 ≤ BMI < 25), overweight (25 ≤ BMI < 30) or obese (BMI ≥ 30).
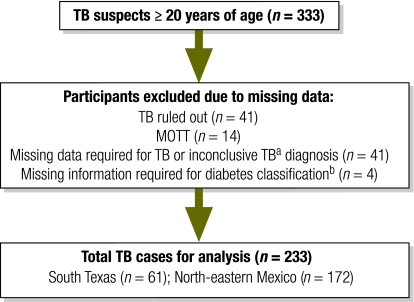
Fig. 1.
Classification of participants in study of the contribution of diabetes to tuberculosis rates, South Texas and north-eastern (NE) Mexico, 2006–2008
MOTT, mycobacteria other than Mycobacterium tuberculosis; TB, tuberculosis.
a Inconclusive TB, smear and culture results unavailable or tuberculosis was diagnosed by physician but there was no documentation of TB treatment for at least 6 months.
b Data required for classification as a diabetic included self-reported diabetes or blood glucose information.
Sources of data from the general population
For South Texas we estimated the prevalence of diabetes among adults 20 years of age or older from our community-based cohort, an ongoing population-based study in Cameron county in which the interview instrument used and the definition of diabetes are the same as in the present TB study.24,27 The incidence of TB and the prevalence of HIV infection were provided by Nita Ngo (personal communication, Texas DSHS) and we obtained the estimated population for 2005–2007 from the United States Census Bureau.30 For north-eastern Mexico we took the prevalence of diabetes from a study conducted in the United States-Mexico border population.25 Statistics for HIV infection and TB and the population size for the city of Matamoros were provided by JL Robles and JS Hernández, SSA-Tamaulipas (personal communication) and from published data.25,31
Data analysis
The study design followed STROBE guidelines.32 We used two-sample t-tests to compare continuous variables and χ2 or Fisher’s exact tests for categorical variables. Given the low incidence of TB and HIV infection in our study populations and because our estimation of odds ratios and relative risks (RRs) yielded similar numbers, we report RRs in this study. For each specific exposure variable (diabetes or HIV infection), the association with TB is expressed as an RR using the following formula:
We calculated the prevalence of exposure to diabetes among new TB cases, p(exposed|new TB cases) directly from our study data, and we obtained the prevalence of exposure to diabetes among the general population, p(exposed), from the aforementioned surveillance data. To calculate the 95% CI of the RR, we first calculated the exact binomial CI of p(exposed|new TB cases), denoted by (r1, r2). P(exposed) is considered a constant because it is estimated with a much larger sample size. The 95% CI of the RR is then given by (r1/(1−r1) × (1−p(exposed))/p(exposed), r2/(1−r2) × (1−p(exposed))/p(exposed)). The attributable risk among general population, ARpopulation, is defined as the proportion of incidence rate of TB due to diabetes or HIV infection in the general population that would be reduced if the exposure were eliminated and is estimated by ARpopluation = p(exposed)(RR−1)/[1 + p(exposed)(RR−1)]. The attributable risk among the exposed (to diabetes or HIV infection in our study), ARexposed, is defined as the reduction in the incidence of TB among the exposed population that would be experienced if the exposure were eliminated. It is estimated by ARexposed = 1−1/RR. Data were analysed with SAS version 9.1 (SAS Institute, Cary, USA). Significance was set at P ≤ 0.05 and marginal significance at P ≤ 0.10.
Results
Characteristics of enrolled patients
A total of 333 TB suspects were enrolled in the study, and 233 fulfilled the criteria for analysis: 61 in South Texas and 172 in north-eastern Mexico (Fig. 1). All participants had pulmonary TB: 109 (46.8%) were confirmed by culture (55 in South Texas and 54 in north-eastern Mexico), 118 (50.6%) had a positive sputum smear (3 in South Texas and 115 in north-eastern Mexico) and 6 (2.6%) had clinical TB (3 in South Texas and 3 in north-eastern Mexico). Most TB patients were born in Mexico. Nearly all were Hispanics and more than 60% were male (Table 1). Patients from north-eastern Mexico were younger, more likely to be employed and less educated than those from South Texas. Mexican patients were more likely to abuse alcohol or consume illicit drugs than South Texas patients. Cigarette smoking was reported by one third of the 170 participants (34% Texas; 29% Mexico; data not shown). HIV infection rates were low. South Texas patients had a higher average BMI. Diabetes was common in patients from both countries: 39.% in South Texas and 36% in north-eastern Mexico (Table 1). Among diabetes patients, those from north-eastern Mexico were less likely to be aware that they had diabetes before this study [1/25 (4.2%) in South Texas, 12/62 (19.4%) in north-eastern Mexico)], a finding more common among males (12 males versus 1 female, P = 0·03). More than two thirds of the diabetes patients had chronic hyperglycaemia.
Table 1. Characteristics of tuberculosis patients, South Texas and north-eastern Mexico, 2006–2008a.
| Host characteristic | South Texas (n = 61) | NE Mexico (n = 172) | P |
|---|---|---|---|
| Demographic | |||
| Age in years, mean (± SD) | 47 (17) | 43 (16) | 0.07 |
| Female gender | 23 (38) | 51 (30) | 0.26 |
| Hispanic ethnicity | 61 (100) | 172 (100) | NA |
| White race | 61 (100) | 172 (0) | NA |
| Country of birth | < 0.0001 | ||
| Texas | 18 (30) | 1 (1) | |
| Mexico | 43 (71) | 171 (99) | |
| Education | 0.0002 | ||
| None | 12 (20) | 19 (11) | |
| Elementary or middle school | 27 (44) | 126 (73) | |
| High school or higher | 22 (36) | 27 (16) | |
| Employed | 34 (56) | 122 (71) | 0.04 |
| Mean annual household income (in US$)b | |||
| Quartile 1 | 6 516 | 2 640 | NAc |
| Quartile 2 | 10 370 | 3 927 | NAc |
| Quartile 3 | 17 520 | 5 232 | NAc |
| Quartile 4 | 88 000 | 57 600 | NAc |
| Known risk factors for tuberculosis | |||
| Alcohol abuse | 5 (8) | 36 (21) | 0.03 |
| Drug abuse | 8 (13) | 43 (25) | 0.07 |
| History of incarceration | 4 (7) | 11 (6) | 1.00 |
| Positivity to HIV | 2 (3) | 7 (4) | 1.00 |
| Diabetes and associated conditions | |||
| Diabetes patients | 24 (39) | 62 (36) | 0.65 |
| Previously undiagnosed diabetics | 1 (4) | 12 (19) | 0.10 |
| Chronic hyperglycaemia (HbA1c ≥ 6.5%) | 18 (82) | 34 (64) | 0.17 |
| HbA1c, mean (± SD) | 8.7% (2) | 8% (3) | 0.20 |
| BMI | < 0.0001 | ||
| Underweight | 4 (7) | 42 (25) | |
| Normal weight | 31 (51) | 98 (57) | |
| Overweight | 26 (43) | 31 (18) |
BMI, body mass index; HbA1c, glycosylated haemoglobin; HIV, human immunodeficiency virus; NA, not applicable; SD, standard deviation.
a All values represent the absolute number followed by the percentage in paretheses unless otherwise indicated. Due to missing information, totals may not always add up to the sample size. Percentages have been rounded.
b 11 Mexican pesos to 1 US$.
c Not applicable because of differences in currency between study sites.
Reference populations for diabetes prevalence
The prevalence of diabetes among South Texas adults was estimated at 19.5%.24 Cameron county cohort participants resembled TB patients in their age distribution, ethnicity, Mexican origin (66%), low education and socioeconomic status. The self-reported average annual household incomes, by quartiles, were $7470, $12 000, $20 000 and $300 000. In contrast, the cohort had a higher proportion of females (67%) and obese individuals (60%).
There were three possible sources of diabetes prevalence estimates from the general population in north-eastern Mexico: (i) a nationwide study conducted in 2000 (9.5%);33 (ii) a report from a diabetes prevention programme on the United States-Mexico border conducted from 2001–2002 data (15.1%);25,31 and (iii) unpublished data from the SSA-Tamaulipas (15%). Although they did not provide detailed sociodemographic data for the Mexican participants, we used the last two sources because their figures were similar and more recent and because they would yield a more conservative estimate of attributable risk. For the United States-Mexico border study, combined statistics for the 4027 participants from both sides of the border (2122 from Mexican states, among these 331 from Tamaulipas) showed a relatively homogeneous age distribution (nearly 20% in each age group), a predominance of females (71%) and high rates of overweight (37%) and obesity (38%).
TB risk attributable to diabetes or HIV infection
The prevalence of diabetes was significantly higher among TB patients from the two study sites than among the corresponding general populations (39.3% versus 19.5% in South Texas; 36.0% versus 15.1% in north-eastern Mexico).24,25 Taking into account these statistics and the TB incidence among adults(18 per 100 000 in South Texas and 32 per 100 000 in north-eastern Mexico), we estimated that in both countries diabetes patients had three times the risk of developing TB as non-diabetics and that those aged 35 to 64 years had five times the risk (Table 2; no data for north-eastern Mexico). The estimated fraction of TB cases attributable to diabetes in adults 20 years old or older was 26% in South Texas and 24% in north-eastern Mexico (Table 2). In South Texas, this figure increased to 48% among individuals 35–64 years old. The fraction of TB cases attributable to diabetes across the population was 63% in South Texas and 68% in north-eastern Mexico.
Table 2. Risk of tuberculosis attributable to diabetes or to HIV infection in study sites in South Texas and north-eastern (NE) Mexico, 2006–2008.
| Age (years) | Diabetes |
HIV infection |
|||||
|---|---|---|---|---|---|---|---|
| RR (95% CI) | ARexposed (%)a | ARpopulation (%)b | RR (95% CI) | ARexposed (%)a | ARpopulation (%)b | ||
| South Texas | |||||||
| 20+ (n = 61) | 2.7 (1.6–4.4) | 63 | 26 | 17.8 (6.5–9.0) | 94 | 5 | |
| 20–34 (n = 20) | 0.9 (0.1–6.8) | −9 | 1 | 34.4 (8.0–147.7) | 97 | 6 | |
| 35–64 (n = 32) | 5.1 (2.6–10.2) | 80 | 48 | 12.2 (2.9–50.9) | 92 | 5 | |
| 65+ (n = 9) | 1.7 (0.5–5.8) | 41 | 22 | 0c | NA | NA | |
| NE Mexico | |||||||
| 20+ (n = 172) | 3.1 (2.3–4.2) | 68 | 24 | 16.0 (7.5–34.0) | 94 | 3 | |
AR, attributable risk; CI, confidence interval; HIV, human immunodeficiency virus; NA, not applicable; RR, relative risk.
a Risk of tuberculosis attributable to exposure among exposed patients, ARexposed = 1 – 1/RR.
b Risk of tuberculosis attributable to exposure among the corresponding general population, ARpopulation = p(exposed)(RR − 1)/[1 + p(exposed)(RR − 1)].
c There were no HIV-positive patients in this age group.
Based on the official prevalence of HIV infection (0.19% for South Texas and 0.24% for north-eastern Mexico), similar calculations for HIV infection indicated that the contribution of this exposure to TB was ≤ 5% at the population level and 94% among HIV-positive individuals in both countries. Altogether, these data indicate that HIV infection is a major risk factor at the individual level, but that diabetes has a larger impact on TB at the population level.
TB patients with diabetes
Given the important contribution of diabetes to TB incidence, we further characterized TB patients to define the profile of those with diabetes. To do this we merged the data sets from both countries. TB patients with diabetes were more likely to be older and overweight or obese and less likely to abuse alcohol or drugs (Table 3). Smoking did not differ significantly between TB patients with diabetes or without it (24% and 34%, respectively) [(odds ratio: 0.6; 95% CI: 0.3–1.2), data not shown].
Table 3. Sociodemographic characteristics, by diabetes status, of tuberculosis patients in South Texas and north-eastern (NE) Mexico, 2006–2008.
| Characteristica | Diabetes (n = 86) | No diabetes (n = 147) | P |
|---|---|---|---|
| Country of enrolment | 0.65 | ||
| United States | 24 (28) | 37 (25) | |
| Mexico | 62 (72) | 110 (75) | |
| Age Mean (± SD) | 49.9 (13) | 40.3 (17) | < 0.0001 |
| Female gender | 32 (37) | 42 (29) | 0.17 |
| Education | 0.70 | ||
| None | 13 (15) | 18 (12) | |
| Elementary or middle school | 57 (66) | 96 (65) | |
| High school or higher | 16 (19) | 33 (22) | |
| Employed | 53 (62) | 103 (70) | 0.19 |
| BMI | 0.0001 | ||
| Underweight | 10 (12) | 36 (25) | |
| Normal weight | 42 (50) | 87 (60) | |
| Overweight/obese | 32 (38) | 23 (16) | |
| Alcohol abuse | 9 (11) | 32 (22) | 0.03 |
| Drug abuse | 11 (13) | 40 (27) | 0.01 |
| History of incarceration | 2 (2) | 13 (9) | 0.06 |
| HIV+ | 0 (0) | 9 (6) | 0.03 |
BMI, body mass index; HIV, human immunodeficiency virus; SD, standard deviation.
a All values represent the absolute number followed by the percentage in paretheses unless otherwise indicated. Due to missing information, totals may not always add up to the sample size. Percentages have been rounded.
Although diabetes is a recognized risk factor for TB, whether TB induces a transient hyperglycaemia that would be classified as “diabetes” remains controversial.34 We found that patients with diabetes had been aware of having diabetes for approximately 8 years on average before their TB diagnosis, and they were more likely to report co-morbidities classically associated with diabetes than patients without diabetes (data not shown). These data suggest that TB developed in patients who already had diabetes.
Discussion
Our study shows that the prevalence of diabetes among TB patients from adjacent populations in South Texas and north-eastern Mexico is very high and among the highest in the world.6–17 One quarter of the cases of TB in these medically-underserved communities along the Texas–Mexico border were attributable to diabetes. With diabetes on the rise in TB-endemic areas, our findings highlight the re-emerging impact of diabetes, now type 2, on TB control in regions of the world where both diseases are prevalent.
Our study was designed to estimate the contribution of diabetes to TB. To accurately define diabetes, we used primary data collected prospectively from newly-diagnosed TB patients. However, the study has some limitations. First, we may have overestimated the prevalence of diabetes because TB can cause transient hyperglycaemia.8,35 If so, our margin of error is ≤ 3% based on the concordance between our current definition based on hyperglycaemia as well as self-reported diabetes and the stricter definition based solely on chronic hyperglycaemia (HbA1c ≥ 6.5%; kappa 0.93; 95% CI: 0.88–0.98).
Second, inherent biases in data sets may have affected the comparison of TB patient data with data from the corresponding adult populations. We carefully selected age-matched data from official health department statistics or research studies in which diabetes was confirmed with blood tests. For South Texas the methods used to characterize the general population and TB patients were nearly-identical, but sociodemographic data for Mexico were less detailed. The data suggest that in both countries the proportion of females and the average BMI were higher in the general population than among TB patients, not surprisingly since TB is more prevalent among males and can cause dramatic weight loss. In any event, even if obesity in the general populations were overrepresented, this would lead to an underestimation of the TB risk attributable to diabetes because people with obesity are more prone to diabetes than people of normal weight.2,27
A third limitation comes from the assumptions made in calculating attributable risk: that a causal association exists between diabetes and TB and that other risk factors for TB are equally distributed among TB patients with and without diabetes. In our study, known risk factors for TB were less frequent among TB patients with diabetes (Table 3). Therefore, our attributable risk calculations may be underestimates of the contribution of diabetes to TB. Finally, M. tuberculosis infection was not confirmed by culture in a substantial number of participants. Nevertheless, we are now conducting routine culture on all TB suspects and find that only 0.5% of smear-positive cases have atypical mycobacteria.
Nearly all our diabetes patients (particularly in South Texas) were aware of having diabetes for at least 6 months before being diagnosed with TB. These findings reveal that diabetic patients with TB are not new to the health-care system and highlight the fact that opportunities for preventing TB among diabetes patients are often missed. While not all diabetes patients with latent TB infection should take prophylactic treatment,36 such patients should be made aware of their risk of TB and should discuss with their physicians the potential risks and benefits of taking preventive anti-TB treatment. Our findings and what is currently known about the natural history of TB suggest that people with a history of diabetes and its complications who have had recent contact with a TB patient are prime candidates for preventive treatment. This would involve screening contacts for diabetes during contact investigations, a measure that would be most cost-efficient if only contacts 35 years or older were targeted. In our setting, diabetic individuals 35–64 years of age accounted for nearly 50% of the TB cases in this age group. These measures will also make it necessary to update the educational curriculum of health professionals to increase their awareness of the re-emerging association between TB and diabetes.
The impact of diabetes on TB control varies by country. In the United States TB rates are disproportionately higher among Hispanics, African Americans and American Indians, all of whom are also at higher risk for diabetes. Further research on the contribution of diabetes to TB in these populations is needed.7,37,38 In other regions of the world the contribution of diabetes to TB is largely unknown Of the seven countries that have the highest numbers of TB and diabetes patients in the world, India is the only one for which the risk of TB attributable to diabetes has been reported, based on secondary data. The results suggest an important contribution (20% for smear-positive TB)13 that warrants further investigation.
Our findings suggest that in Mexico the fraction of diabetics who know they have the illness is lower than in South Texas, especially among males, and the same may be true in other developing countries. Better access to health care in the United States may explain why awareness is higher, even though South Texas is one of the country’s poorest regions.30 This finding points to the potential benefits of partially integrating TB and diabetes control programmes worldwide. Males may benefit the most given the higher prevalence of TB among them39 and the fact that the acute symptoms of TB often motivate them to present to the health system. In contrast, type 2 diabetes is insidious and can persist for years without diagnosis when access to medical care is limited.40 Delayed diagnosis occurs when patients present with complications from chronic hyperglycaemia. The infrastructure for TB control could serve to improve the early detection of diabetes patients, particularly in developing countries. In light of these findings, diabetes control worldwide could be greatly enhanced by the sharing of resources, experience and infrastructure among resource-limited programmes for TB and diabetes control.41
Acknowledgements
We thank the following for their support: Diana Gomez, Perla Martinez, Caroline Mullin, Paula Pino, Nancy Rouse, and Mary Walsh from the University of Texas School of Public Health, Brownsville campus; Nita Ngo and Brian Smith from the Texas DSHS, and José Luis Robles and Jorge Sebastián Hernández from the SSA-Tamaulipas; Eduardo Olivarez, Gloria Salinas, Lydia Serna and Richard Wing from the Hidalgo County Health Departments; Herminia Fuentes, Raul Loera, Olga Ramos and the staff at the Secretaría de Salud de Matamoros; and Yvette Salinas and the staff from the Cameron County Department of Health and Human Services.
Funding:
Support for this work was provided by NIH NIAID 1 R21 AI064297-01-A1, NIH NCMHD P20 MD000170-04, NIH CCTS-CTSA 1U54RR023417-01 and UL1 RR024148.
Competing interests:
None declared.
References
- 1.Global tuberculosis report Geneva: World Health Organization; 2010. Available from: http://whqlibdoc.who.int/publications/2010/9789241564069_eng.pdf [accessed 25 March 2011].
- 2.Diabetes atlas Brussels: International Diabetes Federation; 2010. Available from: http://www.diabetesatlas.org/ [accessed 25 March 2011].
- 3.Dye C. Global epidemiology of tuberculosis. Lancet. 2006;367:938–40. doi: 10.1016/S0140-6736(06)68384-0. [DOI] [PubMed] [Google Scholar]
- 4.Oscarsson PN, Silwer H. Incidence of pulmonary tuberculosis among diabetics. Acta Med Scand. 1958;335:23–48. [PubMed] [Google Scholar]
- 5.Root HF. The association of diabetes and tuberculosis. N Engl J Med. 1934;210:1–13. doi: 10.1056/NEJM193401042100101. [DOI] [Google Scholar]
- 6.Kim SJ, Hong YP, Lew WJ, Yang SC, Lee EG. Incidence of pulmonary tuberculosis among diabetics. Tuber Lung Dis. 1995;76:529–33. doi: 10.1016/0962-8479(95)90529-4. [DOI] [PubMed] [Google Scholar]
- 7.Mori MA, Leonardson G, Welty TK. The benefits of isoniazid chemoprophylaxis and risk factors for tuberculosis among Oglala Sioux Indians. Arch Intern Med. 1992;152:547–50. doi: 10.1001/archinte.152.3.547. [DOI] [PubMed] [Google Scholar]
- 8.Mugusi F, Swai AB, Alberti KG, McLarty DG. Increased prevalence of diabetes mellitus in patients with pulmonary tuberculosis in Tanzania. Tubercle. 1990;71:271–6. doi: 10.1016/0041-3879(90)90040-F. [DOI] [PubMed] [Google Scholar]
- 9.Pablos-Méndez A, Blustein J, Knirsch CA. The role of diabetes mellitus in the higher prevalence of tuberculosis among Hispanics. Am J Public Health. 1997;87:574–9. doi: 10.2105/AJPH.87.4.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coker R, McKee M, Atun R, Dimitrova B, Dodonova E, Kuznetsov S, et al. Risk factors for pulmonary tuberculosis in Russia: case-control study. BMJ. 2006;332:85–7. doi: 10.1136/bmj.38684.687940.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pérez A, Brown HS, 3rd, Restrepo BI. Association between tuberculosis and diabetes in the Mexican border and non-border regions of Texas. Am J Trop Med Hyg. 2006;74:604–11. [PMC free article] [PubMed] [Google Scholar]
- 12.Shetty N, Shemko M, Vaz M, D’Souza G. An epidemiological evaluation of risk factors for tuberculosis in South India: a matched case control study. Int J Tuberc Lung Dis. 2006;10:80–6. [PubMed] [Google Scholar]
- 13.Stevenson CR, Forouhi NG, Roglic G, Williams BG, Lauer JA, Dye C, et al. Diabetes and tuberculosis: the impact of the diabetes epidemic on tuberculosis incidence. BMC Public Health. 2007;7:234. doi: 10.1186/1471-2458-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung CC, Lam TH, Chan WM, Yew WW, Ho KS, Leung GM, et al. Diabetic control and risk of tuberculosis: a cohort study. Am J Epidemiol. 2008;167:1486–94. doi: 10.1093/aje/kwn075. [DOI] [PubMed] [Google Scholar]
- 15.Alisjahbana B, van Crevel R, Sahiratmadja E, den Heijer M, Maya A, Istriana E, et al. Diabetes mellitus is strongly associated with tuberculosis in Indonesia. Int J Tuberc Lung Dis. 2006;10:696–700. [PubMed] [Google Scholar]
- 16.Ponce-De-Leon A, Garcia-Garcia ML, Garcia-Sancho MC, Gomez-Perez FJ, Valdespino-Gomez JL, Olaiz-Fernandez G, et al. Tuberculosis and diabetes in southern Mexico. Diabetes Care. 2004;27:1584–90. doi: 10.2337/diacare.27.7.1584. [DOI] [PubMed] [Google Scholar]
- 17.Restrepo BI, Fisher-Hoch SP, Crespo JG, Whitney E, Perez A, Smith B, et al. Nuevo Santander Tuberculosis Trackers Type 2 diabetes and tuberculosis in a dynamic bi-national border population. Epidemiol Infect. 2007;135:483–91. doi: 10.1017/S0950268806006935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Restrepo BI, Fisher-Hoch SP, Pino PA, Salinas A, Rahbar MH, Mora F, et al. Tuberculosis in poorly controlled type 2 diabetes: altered cytokine expression in peripheral white blood cells. Clin Infect Dis. 2008;47:634–41. doi: 10.1086/590565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stalenhoef JE, Alisjahbana B, Nelwan EJ, van der Ven-Jongekrijg J, Ottenhoff TH, van der Meer JWM, et al. The role of interferon-gamma in the increased tuberculosis risk in type 2 diabetes mellitus. Eur J Clin Microbiol Infect Dis. 2008;27:97–103. doi: 10.1007/s10096-007-0395-0. [DOI] [PubMed] [Google Scholar]
- 21.Martens GW, Arikan MC, Lee J, Ren F, Greiner D, Kornfeld H. Tuberculosis susceptibility of diabetic mice. Am J Respir Cell Mol Biol. 2007;37:518–24. doi: 10.1165/rcmb.2006-0478OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vallerskog T, Martens GW, Kornfeld H. Diabetic mice display a delayed adaptive immune response to Mycobacterium tuberculosis. J Immunol. 2010;184:6275–82. doi: 10.4049/jimmunol.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Global tuberculosis control: surveillance, planning, financing: WHO report 2009 Geneva: world Health Organization; 2009.
- 24.Fisher-Hoch SP, Rentfro AR, Salinas JJ, Pérez A, Brown HS, Reininger BM, et al. Socioeconomic status and prevalence of obesity and diabetes in a Mexican American community, Cameron County, Texas, 2004–2007. Prev Chronic Dis. 2010;7:A53. [PMC free article] [PubMed] [Google Scholar]
- 25.The U. S.-Mexico Border Diabetes Prevention and Control Project: first report of results Washington: Pan American Health Organization; 2005. [Google Scholar]
- 26.Global tuberculosis control: surveillance, planning, financing: WHO report 2008 Geneva: World Health Organization; 2008. [Google Scholar]
- 27.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;32(Suppl 1):S62–7. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan DM, Peterson CM, et al. American Diabetes Association Tests of glycemia in diabetes. Diabetes Care. 2004;27(Suppl 1):S91–3. doi: 10.2337/diacare.27.7.1761. [DOI] [PubMed] [Google Scholar]
- 29.Rehm J, Greenfield TK, Walsh G, Xie X, Robson L, Single E. Assessment methods for alcohol consumption, prevalence of high risk drinking and harm: a sensitivity analysis. Int J Epidemiol. 1999;28:219–24. doi: 10.1093/ije/28.2.219. [DOI] [PubMed] [Google Scholar]
- 30.State and county quick facts: Hidalgo county, Texas Washington: US Census Bureau; 2010. Available from: http://quickfacts.census.gov/qfd/states/48/48215.html [accessed 25 March 2011].
- 31.Gibson DV, Rhi-Perez P, Zavaleta A. At the crossroads: the Texas/Mexico border economy at Cameron County and Matamoros. Texas Business Review (University of Texas at Austin) 2006 June.
- 32.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 33.Encuesta Nacional de Salud 2000 Mexico City: Instituto Nacional de Salud Pública & Secretaría de Salud de México; 2000. Spanish. Available from: http://www.insp.mx/ensa/ensa_tomo2.pdf#search='Encuesta%20Nacional%20Salud%20Mexico%202000' [accessed 25 March 2011].
- 34.Koziel H, Koziel MJ. Pulmonary complications of diabetes mellitus. Pneumonia. Infect Dis Clin North Am. 1995;9:65–96. [PubMed] [Google Scholar]
- 35.Umeki S. Glucose intolerance in chronic respiratory failure. Angiology. 1994;45:937–42. doi: 10.1177/000331979404501105. [DOI] [PubMed] [Google Scholar]
- 36.Harries AD, Murray MB, Jeon CY, Ottmani SE, Lonnroth K, Barreto ML, et al. Defining the research agenda to reduce the joint burden of disease from diabetes mellitus and tuberculosis. Trop Med Int Health. 2010;15:659–63. doi: 10.1111/j.1365-3156.2010.02523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diabetes: disabling, deadly and on the rise. The Diabetes Monitor 2005 April 28. Available from: http://www.diabetesmonitor.com/b324.htm [accessed 25 March 2011].
- 38.Umpierrez GE, Gonzalez A, Umpierrez D, Pimentel D. Diabetes mellitus in the Hispanic/Latino population: an increasing health care challenge in the United States. Am J Med Sci. 2007;334:274–82. doi: 10.1097/MAJ.0b013e3180a6efe3. [DOI] [PubMed] [Google Scholar]
- 39.Iademarco MF, Castro KG. Epidemiology of tuberculosis. Semin Respir Infect. 2003;18:225–40. doi: 10.1053/S0882-0546(03)00074-4. [DOI] [PubMed] [Google Scholar]
- 40.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31(Suppl 1):S55–60. doi: 10.2337/dc08-S055. [DOI] [PubMed] [Google Scholar]
- 41.Harries AD, Billo N, Kapur A. Links between diabetes mellitus and tuberculosis: should we integrate screening and care? Trans R Soc Trop Med Hyg. 2009;103:1–2. doi: 10.1016/j.trstmh.2008.08.008. [DOI] [PubMed] [Google Scholar]