Figure 6.
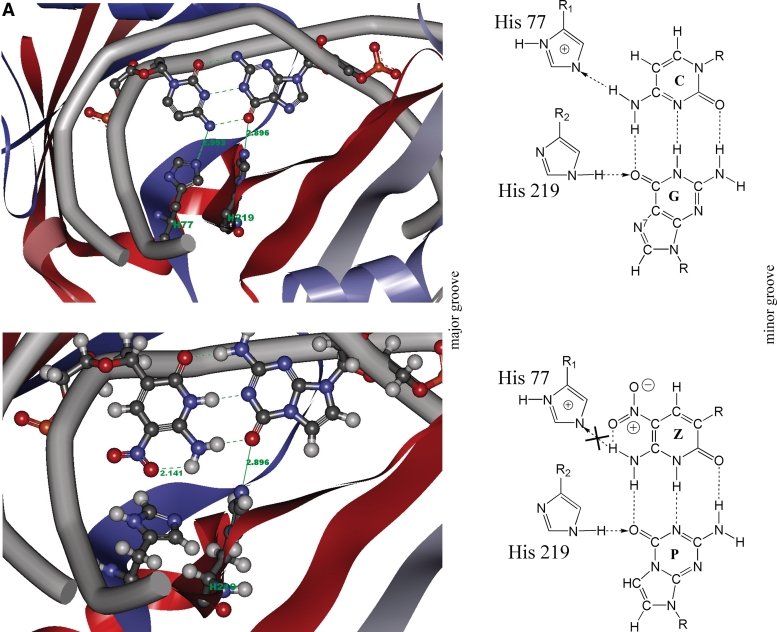
(A1) Detailed diagram of the hydrogen bonding interactions between the central C–G (upper panel) or Z–P (lower panel) base pair (from major groove) and His 77, His 219 of BcnI (PDB: 2Q10). The hydrogen bonds are marked by green lines and their distances are labeled in green numbers. The atoms are colored by element. The lower panel shows that the oxygen atom of the nitro group of Z forms intramolecular hydrogen bond with its exocyclic NH, which disrupts the intermolecular hydrogen bond contacting to His 77. (A2) Schematic showing recognition of the central base pair from major groove with His 77 and His 219 of BcnI (recognition sequence: CCSGG). The upper panel shows the hydrogen bonding interactions between the central C–G base pair and His 77, 219 of BcnI. The arrow indicates the hydrogen-bond between donor and acceptor. The lower panel shows the hydrogen bonding interactions between the central Z–P base pair and His 77, 219 of BcnI. Here the nitro group of Z forms intramolecular hydrogen bond with its exocyclic NH. As a result, it disrupts the intermolecular hydrogen bond contacting to His 77 (indicated as cross). (B1) Detailed diagram of the hydrogen bonding interactions between the second (or sixth, by palindromy) C–G (upper panel) or Z–P (lower panel) base pair and Trp 130, Lys 173, Leu 134 of EcoO109I (PDB: 1WTE). The hydrogen bonds are marked by green lines and their distances are labeled in green numbers. H2O is presented as red sphere and the atoms are colored by element. In the lower panel, the hydrogen bond contacting to Trp 130 is retained, because the exocyclic NH of Z and the oxygen atom of acyl group of Trp 130 lie on one line. This is beneficial to the energy minimization and structural stability. On the contrary, the hydrogen bond contacting to Leu 134 is disrupted due to the substituent at 7 position of P. (B2) Schematic showing recognition of the second or sixth (by palindromy) base pair from major groove with Trp 130, Lys 173, Leu 134 of EcoO109I (recognition sequence: RGGNCCY). The upper panel shows the hydrogen bonding interactions between the second (or sixth) C–G base pair and EcoO109I. The arrow indicates the hydrogen-bond between donor and acceptor. The lower panel shows the hydrogen bonding interactions between the second (or sixth) Z–P base pair and EcoO109I. The hydrogen bond between Trp 130 and Z is retained, whereas the hydrogen bond between Leu 134 and P is disrupted (indicated as cross). (C1) Detailed diagram of the hydrogen bonding interactions between the second (or seventh) C–G (upper panel) or Z–P (lower panel) base pair and Asn 230, His 189, Gly 190 of NotI (PDB:3C25). The hydrogen bonds are marked by green lines and their distances are labeled in green numbers. The atoms are colored by element. Since the space steric hindrance of the nitro group of Z increases the distance between of the exocyclic amide-N and the oxygen of side chain carbonyl group of Asn 230, the hydrogen bond contacting to Asn 230 is disrupted. The hydrogen bond contacting to Gly 190 is also disrupted because of the substituent at 7 position of P. (C2) Schematic showing recognition of the second or seventh (symmetric) base pair from major groove with Asn 230, His 189, Gly 190 of NotI (recognition sequence: GCGGCCGC). The upper panel shows the hydrogen bonding interactions between the second or seventh C–G base pair and NotI. The arrow indicates the hydrogen-bond between donor and acceptor. The lower panel shows the hydrogen bonding interactions between the second or seventh Z–P base pair and NotI. The hydrogen bond contacting to Asn 230 and Gly 190 are destroyed (indicated as cross).