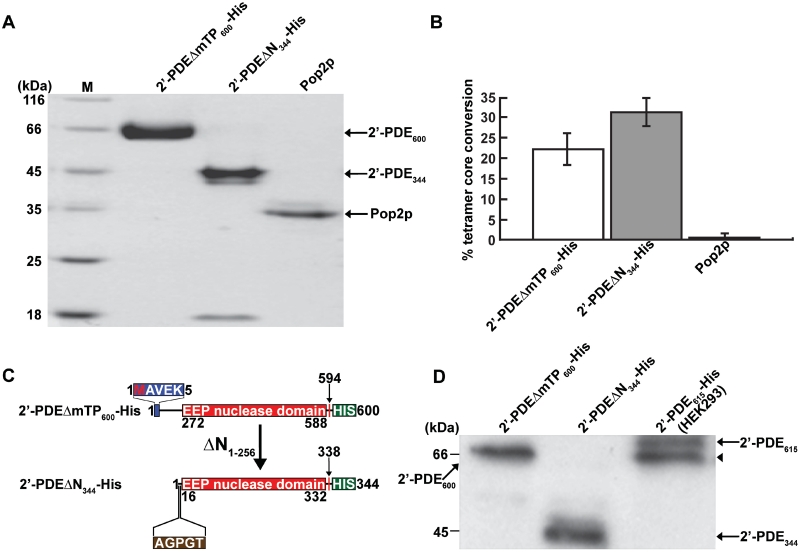
Figure 5.
Purification and validation of 2′-PDE expressed in E. coli BL21(DE3). (A) Coomassie blue-stained gel after SDS–PAGE showing 20 µg of purified 2′-PDEΔmTP600-His and 2′-PDEΔN344-His. Pop2p is a purified DEDD-type deadenylase from Schizosaccharomyces pombe used as a control in (B) (20). Arrows specify the position of the indicated proteins. The M lane contains marker proteins of different molecular masses. (B) 2′–5′ oligoadenylate degradation assay on the purified protein fractions from (A) using a 2′–5′ oligoadenylate tetramer, ApApApA, as substrate. The deadenylase Pop2p is only active on 3′–5′-linked RNA and thus constitutes a negative control for 2′–5′ oligoadenylate degradation. Reactions were performed in triplicate (mean/SEM) using 10 µg of protein. (C) The 2′-PDEΔN344-His truncation fragment and comparison to 2′-PDEΔmTP600-His. The five N-terminally sequenced residues of 2′-PDEΔN344-His are shown (brown box). (D) Immunoblotting of the 2′-PDE proteins from (A) using a His-tag specific antibody (Genscript). A HEK293 total cell lysate expressing the 2′-PDE615-His was employed as a positive control. Arrows denote individual proteins and the arrowhead designates the singly processed form of 2′-PDE615-His present in the HEK293 cell lysate. Of total protein, 10 µg were loaded into each lane.