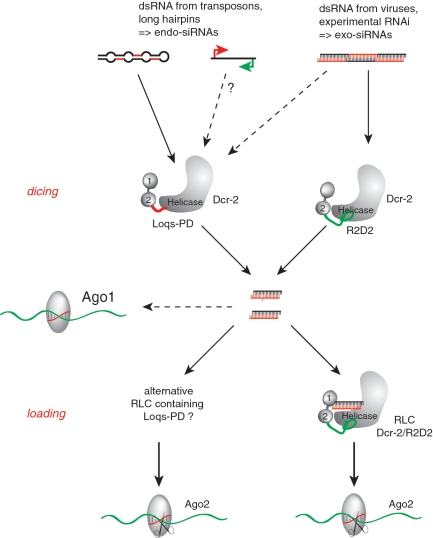
Figure 7.
Distinct biogenesis pathways for exo- and endo-siRNAs. Loqs-PD and R2D2 both contain two N-terminal double-stranded RNA binding domains (labeled 1 and 2) and associate via their C-terminal domain with the N-terminal helicase domain of Dcr-2. This is analogous to the situation of Dcr-1/Loqs-PB and of human Dicer/TRBP or PACT. Despite the similar architecture, the two Drosophila complexes are functionally distinct: Our results indicate that R2D2 and Loqs-PD compete for association with Dcr-2, and that parallel pathways for processing and loading of siRNAs exist. Analogous to the situation for miRNA biogenesis, they appear to be uncoupled after the dicing step. Thus, it is possible that a siRNA is processed by Dcr-2/Loqs-PD and loaded by Dcr-2/R2D2, but this sequence of events is not obligatory. R2D2 may not be required for the dicing reaction; since competition for Dcr-2 exists we propose that the presence of “free” Dcr-2 unlikely. Two recent publications demonstrate that endogenous siRNAs can also be loaded into Ago1 to some extent (49,50).