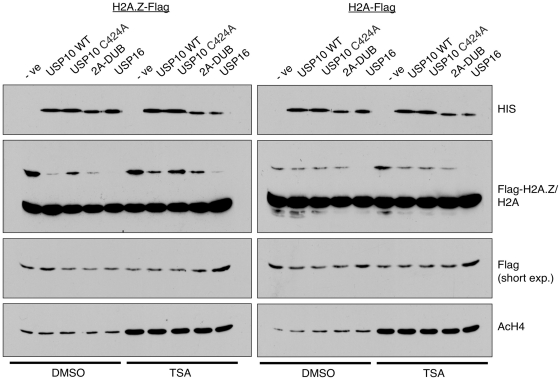
Figure 2.
USP10 deubiquitylates H2A.Zub1 and H2Aub1 in vitro. In vitro deubiquitylation assays were assembled using a panel of His-tagged deubiquitylases that were generated using the baculovirus protein expression system. Purified deubiquitylases were incubated with acid-soluble protein extracts from nuclei of 293T cells expressing either Flag-tagged H2A.Z or H2A. Levels of the various deubiquitylases were compared by Western blotting using an anti-His antibody (top panel) and levels of the mono-ubiquitylated tagged histones were assessed using the Flag M2 antibody—short exposures of the Flag blots show the unmodified band of the tagged histones for loading purposes. In order to determine if hyper-acetylated histones are better substrates for in vitro deubiquitylation, acid-soluble protein was harvested from cells treated with TSA and hyper-acetylation of histones was confirmed using an anti-acetyl H4 antibody (bottom panel). See text for discussion.