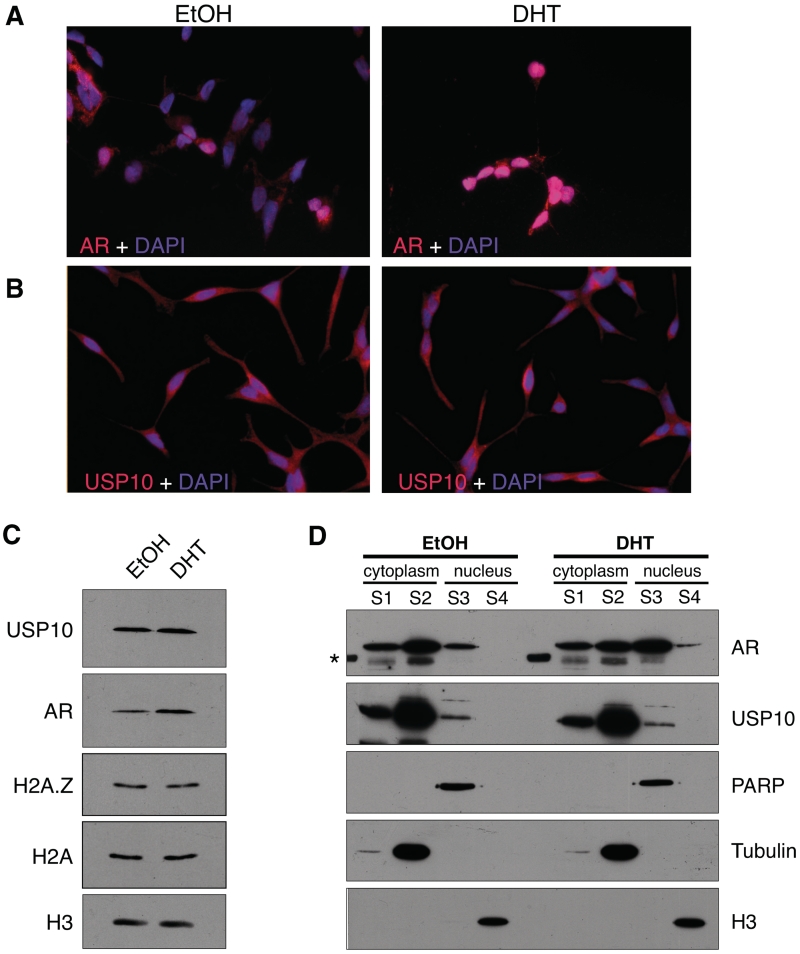
Figure 3.
USP10 localizes to the cytoplasm and nucleus. LNCaP cells were grown on glass coverslips in charcoal-stripped, phenol red-free RPMI 1640 media for 72 h. Cells were then treated for 2 h with either DHT at a final concentration of 10 nM, or an equivalent volume of absolute EtOH. Cells were fixed and co-stained with DAPI (4′,6-diamidino-2-phenylindole) and antibodies recognizing either AR (A) or USP10 (B). (C) Total protein levels of AR and USP10 were compared, via Western blot, between LNCaP cells treated with DHT or EtOH for 2 h (H3 used as a loading control). (D) Sub-cellular localization of USP10 and AR was determined by Western blot using cytoplasmic and nuclear fractions (S1––membrane-associated fraction; S2––soluble cytoplasmic fraction; S3––soluble nuclear fraction; S4––chromatin-associated fraction) of LNCaP cells treated with EtOH or DHT for 2 h. Tubulin, PARP (poly ADP-ribose polymerase) and H3 were used as loading controls for the cytoplasmic, soluble nuclear and chromatin fractions, respectively. Sub-cellular fractions were harvested as described in ‘Materials and Methods’ section. Although AR shows a significant redistribution to the nuclear fraction upon treatment with hormone, USP10 does not redistribute in a similar manner. However, a distinct fraction of USP10 does reside in the nucleus. The asterisk marks a band in the ladder that cross-reacted with the AR antibody.