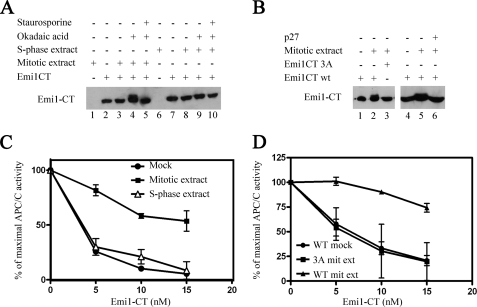
FIGURE 3.
Effect of cell extracts on Emi1 phosphorylation and inhibition of APC/C. A, phosphorylation of recombinant Emi1CT in cell extracts. 10 nm GST-Emi1 C-terminal fragment (Emi1CT) was incubated with the phosphorylation mixture. Where specified, 20 μg of nocodazole-arrested HeLa cell extract (mitotic extract), nuclear extract of HeLa cells 3 h after release from thymidine arrest (S-phase extract), 1 μm okadaic acid, or 10 μm staurosporine were added. Emi1CT phosphorylation was assessed by Western blotting with anti-Emi1 antibody and examination of the Emi1CT electrophoretic mobility shift. B, CDK-dependent phosphorylation of Emi1CT in mitotic cell extracts. 10 nm GST-Emi1CT wild type or mutated in all 3 CDK phosphorylation consensus sites (GST-Emi1CT 3A) was incubated as in A with mitotic extract with or without 0.1 μg/μl of p27, as indicated, and subjected to immunoblotting for Emi1. C, inactivation of Emi1CT by mitotic cell extract. GST-Emi1CT was incubated as described under “Experimental Procedures,” in the presence of phosphorylation buffer alone (●), mitotic extract (■), or S-phase extract (Δ) in duplicates and then purified on glutathione-Sepharose beads as described under “Experimental Procedures.” Following quantitation of these Emi1CT preparations, they were assayed in the indicated concentrations for their ability to inhibit 125I-cyclin B ubiquitylation by APC/CCdc20 in a purified system. The results are expressed as % of maximal APC/C ubiquitin ligase activity. D, effect of mutation of CDK phosphorylation consensus sites of Emi1CT on the inactivation of Emi1CT by mitotic extract. Wild type GST-Emi1CT was incubated as above in the presence of buffer (●) or mitotic extract (▴). GST-Emi1CT 3A was incubated in the presence of mitotic extract (■). Following this incubation, Emi1CT was purified, quantified, and examined for inhibition of APC/C ligase activity as described above.