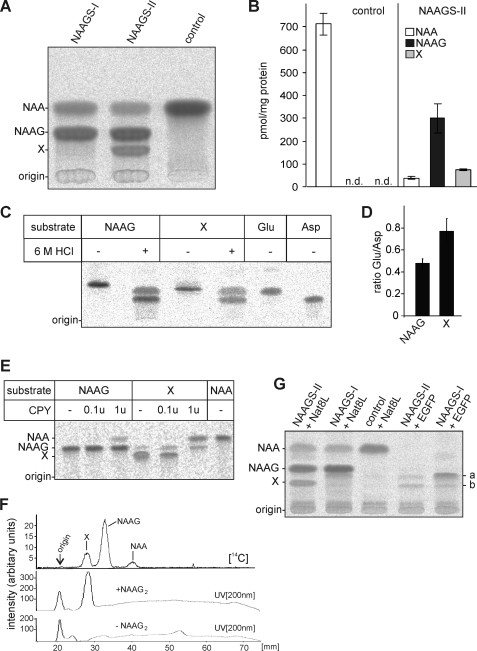
FIGURE 3.
Detection of NAAG synthesis by NAAGS-II. A, CHO-K1 cells transiently cotransfected with plasmids encoding the NAA transporter NaDC3 and NAAGS-I, NAAGS-II, or an irrelevant plasmid (control), were metabolically labeled with [14C]NAA for 16 h. Cells were homogenized in 90% methanol and, after cation exchange chromatography, analyzed by HPTLC. Positions of NAA and NAAG standards are indicated. In contrast to NAAGS-I expressing cells, NAAGS-II expression caused synthesis of an unknown product (X) in addition to the main pre-action product NAAG. B, the amount of NAA, and peptides NAAG and X synthesized in CHO-K1 cells expressing NAAGS-II together with NaDC3, and in control cells, metabolically labeled with [14C]NAA for 16 h were quantified using a Bioimager. Data shown are the mean ± S.D. (n = 3) of three experiments. N.D., not determined. C, HEK-293T cells coexpressing NAAGS-II and Nat8l were metabolically labeled with [14C]glutamate and NAAG and the unidentified products (X) were purified from a methanolic peptide extract by preparative TLC. NAAG and X were subjected to acid hydrolysis in 6 m HCl at 110 °C (+) and left untreated (−). Reaction products were separated by TLC together with 14C-labeled glutamate (Glu) and aspartate (Asp) standards. One representative experiment out of 4 independent experiments is shown. D, the ratio of [14C]Glu to [14C]Asp released by acid hydrolysis shown in C, demonstrated a significant higher Glu content in substance X compared with NAAG (mean ± S.D.; n = 4). E, NAAG and X were isolated as described in C, except that cells were labeled with [14C]NAA. Both substances were treated with increasing amounts of CPY and reaction products were separated by TLC. Although NAAG was a poor substrate for CPY, yielding only small amounts of NAA, peptide X was more efficiently digested by CPY, releasing NAA, and NAAG as an intermediate product (note that substance X could not be completely purified from contaminating NAAG by TLC; the NAAG signal, however, increased during incubation with CPY). F, peptide extracts from NaDC3 and NAAGS-II coexpressing CHO-K1 cells metabolically labeled with [14C]NAA (as shown in panel A) were separated by TLC, in the presence (+NAAG2) or absence (−NAAG2) of synthetic NAAG2, and the distribution of radioactivity was determined using a Bioimager. The position of the internal NAAG2 standard was detected by UV scan at 200 nm. 14C-Labeled substance X and synthetic NAAG2 comigrated. G, metabolic labeling of CHO-K1 cells expressing NAAGS-II or NAAGS-I (cotransfected with or without Nat8l expression plasmids or a control plasmid encoding the EGFP). These experiments showed that additional products (a and b) are synthesized by NAAGS-II and NAAGS-I in the presence and absence of NAA. These products were not detectable in cells transfected with a control and Nat8l expression plasmid.