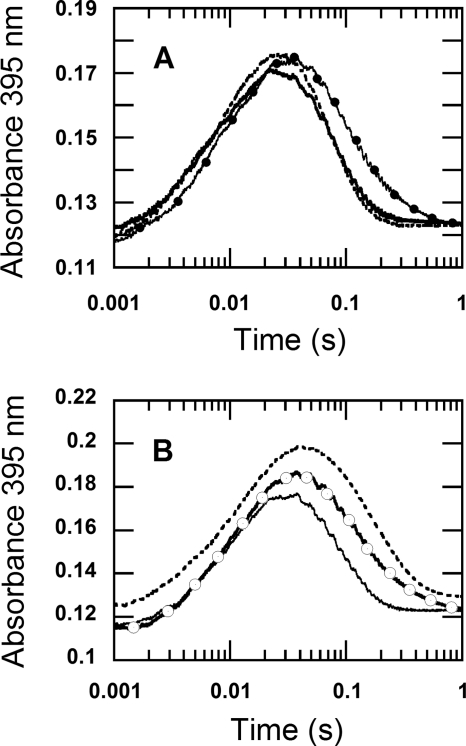
FIGURE 7.
The kinetic isotope effects of the reduced enzyme specifically labeled with deuterium at the flavin N5 position in H2O buffer. A, oxidation of the reduced enzyme labeled with N5-D was investigated using double-mixing stopped-flow spectrophotometry. During the first mixing, a solution of the oxidized enzyme (78 μm before mixing) was mixed with an equal volume of 100 μm 2-d-d-glucose (before mixing) in H2O buffer under anaerobic conditions. The mixture was continued for 80 s after the first mixing, before the reduced enzyme was mixed with 100 mm sodium phosphate buffer containing 0.96 mm oxygen (final concentration) (filled circle line). Only H2O buffers were employed in this experiment. The reaction was monitored at 395 nm to determine the rate constant for the H2O2 elimination from C4a-hydroperoxyflavin. The control experiment, where a solution of 100 μm d-glucose was used instead of 100 μm 2-d-d-glucose, was carried out in a similar fashion and is shown as a solid line trace. The results show that the kinetics of the trace with filled circles (N5-D reduced flavin) was similar to that of the reduced enzyme in D2O buffer reacting with 0.96 mm oxygen in D2O buffer (data in Fig. 2), whereas the kinetics of the double-mixing reaction employing d-glucose (solid line) was similar to the reaction carried out in H2O buffer (dotted line trace, data from Fig. 2). The dotted line trace was multiplied by a factor of 0.7 and offset by + 0.005 absorbance units to bring the absorbance signal to a similar range as the traces from the double-mixing experiment. B, oxidation of the reduced enzyme labeled with N5-D was carried out using a single-mixing stopped-flow spectrophotometer. Equal volumes of solutions of the oxidized enzyme 88 μm in H2O buffer (before mixing) in syringe A and 100 μm 2-d-d-glucose in H2O buffer (before mixing) in syringe B were manually pushed and mixed in syringe D under anaerobic conditions. The reaction in syringe D was allowed to proceed for 80–100 s to obtain the completely reduced P2O labeled with N5-D before the single-mixing stopped-flow experiment took place by mixing syringe D solution with an aerobic buffer in syringe C. The final reaction contained 22 μm of the reduced enzyme specifically labeled with N5-D and 0.96 mm oxygen in 100 mm sodium phosphate in H2O, pH 7.0 (open circle trace). The open circle trace is similar to the kinetic trace from the reduced enzyme in D2O buffer reacting with 0.96 mm oxygen in D2O buffer (Fig. 2). For a reference, the trace from Fig. 2B was multiplied by a factor of 0.66, offset by + 0.044 absorbance units and is shown as a dotted line. The solid line trace was obtained from the control reaction, in which a solution of 100 μm d-glucose was used instead of 100 μm 2-d-d-glucose, and the reaction was carried out in a similar fashion to that for the open circle trace. The results of the single-mixing stopped-flow in Fig. 7B are similar to the results of the double-mixing stopped-flow experiment in Fig. 7A. The data in this figure indicate that the solvent kinetic isotope effect on the H2O2 elimination from C4a-hydroperoxyflavin is mainly contributed by the N5-H bond breaking.