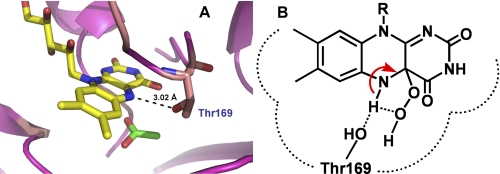
FIGURE 9.
The P2O active site structure. A, the active site of P2O in the closed conformation indicates that the Oγ of Thr169 can make an H-bond interaction with the flavin N5 (29, 30). B, this H-bonding interaction in the wild-type enzyme may divert the intramolecular H-bridge proton transfer, which assists in the H2O2 elimination from C4a-hydroperoxyflavin. Therefore, C4a-hydroperoxyflavin is observed in the wild-type enzyme but not in the Thr169 variants (31).