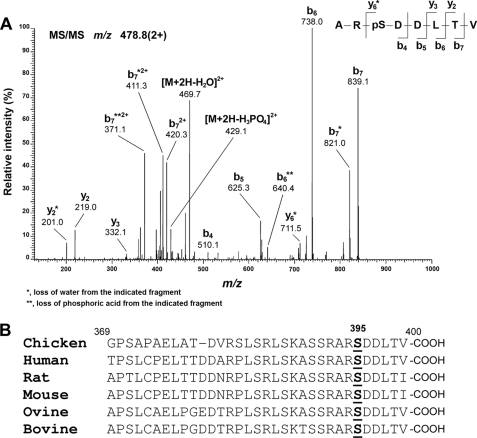
FIGURE 1.
Amino acid residue Ser-395 of Cx50 is phosphorylated in the lens. Cx50 derived from embryonic chick lenses was purified by immunoprecipitation and digested with trypsin before being subjected to LC-MS/MS. A, representative MS/MS spectrum. Assignment of the peptide ARpSDDLTV (Ser(P)-395) is shown. The doubly charged precursor ion, m/z 478.8, was fragmented to produce the spectrum. The observed b-fragment (N-terminal) and y-fragment (C-terminal) ions allowed unambiguous localization of phosphorylation at Ser-395. B, sequence comparison shows that Ser-395 and residues around this site are highly conserved in Cx50 across animal species.