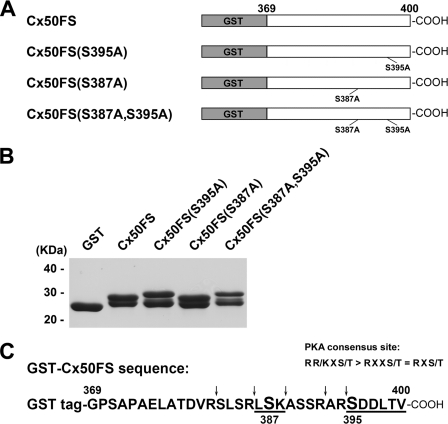
FIGURE 2.
Phosphorylation of GST-tagged Cx50 C terminus fusion proteins and its mutants in vitro by PKA. A, schematic representation of GST-tagged Cx50 C terminus fusion proteins and its mutants GST-Cx50FS(S395A, GST-Cx50FS(S387A), and GST-Cx50FS(S387A,S395A). cDNA fragments encoding a short C-terminal tail (FS, residues 369–400) of Cx50 were subcloned into GST-tagged expression vector pGEX-2T. Site-directed mutagenesis of serine to alanine was performed by a PCR-based mutagenesis method. B, GST-Cx50FS(S395A), GST-Cx50FS(S387A), and GST-Cx50FS(S387A,S395A) were purified and resolved by SDS-PAGE with Coomassie Blue staining. C, amino acid residues 369–400 of Cx50 in the GST fusion protein and predicted tryptic cleavage sites are indicated (arrows). Both Ser-395 and Ser-387 are located within a PKA consensus site (R(R/K)X(S/T) > RXX(S/T) = RX(S/T)). Their corresponding predicted tryptic peptides are underlined.