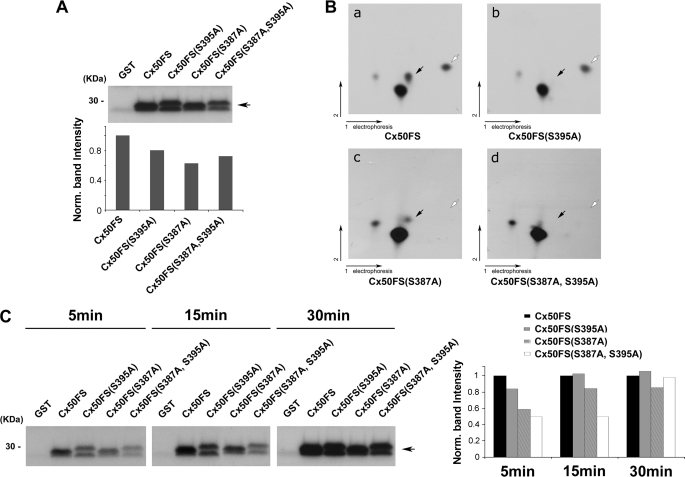
FIGURE 3.
Ser-395 and Ser-387 of Cx50 are phosphorylated by PKA in vitro. A, an autoradiogram showing in vitro PKA phosphorylation of GST-tagged Cx50 C terminus fusion proteins. Purified GST-Cx50FS, GST-Cx50FS(S395A), GST-Cx50FS(S387A), and GST-Cx50FS(S387A,S395A) were in vitro phosphorylated by PKA. GST protein was used as a negative control. Band intensities from Western blots were quantified using densitometry (National Institutes of Health Image J) (lower panel). B, two-dimensional tryptic phosphopeptide mapping of GST-tagged Cx50 C terminus fusion proteins, Cx50FS (panel a), Cx50FS(S395A) (panel b), Cx50FS(S387A) (panel c), and Cx50(S387A,S395A) (panel d). 32P-Labeled GST-tagged Cx50 C terminus fusion proteins were separated by 10% SDS-PAGE, excised, and digested with TPCK-trypsin. The tryptic peptides were applied to two-dimensional TLC at pH 1.9 and then autoradiographed. The black arrows indicate the tryptic phosphopeptides containing Ser-395, and the white arrows indicate the tryptic phosphopeptides containing Ser-387. C, an autoradiogram showing time course analysis of in vitro PKA phosphorylation. Purified GST-Cx50FS, GST-Cx50FS(S395A), GST-Cx50FS(S387A), and GST-Cx50FS(S387A,S395A) were in vitro phosphorylated by PKA for 5, 15, and 30 min. GST protein was used as a control. Band intensities from Western blots were quantified (right panel).