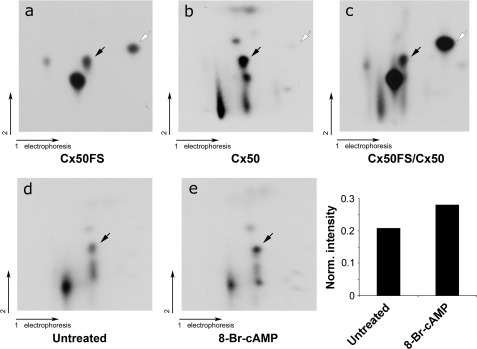
FIGURE 4.
Ser-395 of Cx50 was phosphorylated by PKA in the lens in vivo. Comparison of two-dimensional tryptic phosphopeptide profiles between in vitro phosphorylation of GST-Cx50FS by PKA and in vivo full-length Cx50. 32P-Labeled phosphorylated Cx50 and GST-Cx50FS were separated by SDS-PAGE, excised, and digested with TPCK-trypsin. The tryptic peptides from phosphorylated Cx50 (panel a), GST-Cx50FS (panel b), or mixture of both (panel c) were applied to two-dimensional TLC at pH 1.9. Tryptic phosphopeptides of full-length Cx50 immunoprecipitated from lenses were pretreated (panel e) or untreated (panel d) with 1 mm 8-Br-cAMP. The black arrows indicate the tryptic phosphopeptides containing Ser-395 phosphorylation, and the open arrows indicate the tryptic phosphopeptides containing Ser-387 phosphorylation. Intensities of the dots on panels d and e were measured using densitometry, and the intensity ratio of the spot representing Ser-395 versus all spots is presented (bottom right panel).