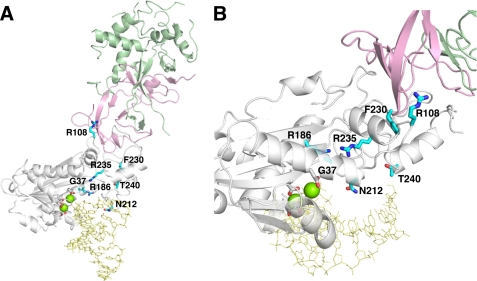
FIGURE 1.
The RNase H2A subunit AGS-causing mutations. A, the mouse RNase H2 heterotrimer with a model RNA-DNA/DNA (yellow) (PDB ID 3KIO (30)) consists of the H2B (green), H2C (pink), and H2A (white) subunits is shown. The human RNase H2A subunit AGS-causing mutations (cyan) were mapped onto the mouse structure. The four conserved acidic residues (white) that likely coordinate two divalent metal ions (green spheres) for catalysis are shown. The indicated residue numbers are of the human H2A protein (actual numbers are mouse:human; Gly-37:Gly-37, Arg-109:Arg-R108, Arg-187:Arg-186, Asn-213:Asn-212, Phe-231:Phe-230, Arg-236:Arg-235, Thr-241:Thr-240). B, the H2A G37S, R186W, and R235Q mutations are in close proximity to the active site with dramatic consequences to catalytic function. The H2A R108W, N212I, F230L, and T240M mutant residues are located more distant from the active site with corresponding reduced effects on nucleic acid binding and catalysis.