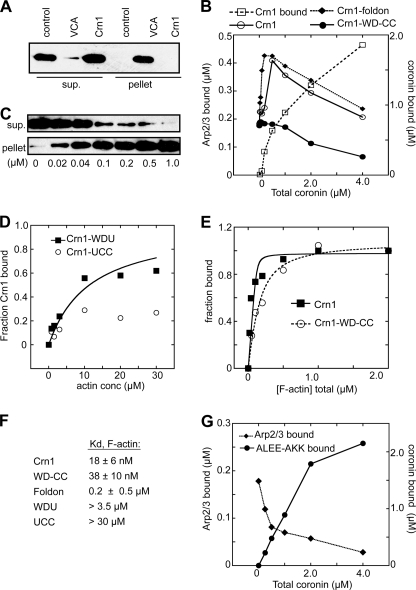
FIGURE 3.
Crn1 recruits Arp2/3 complex to actin filaments. A, GST pulldown assay showing that Crn1 does not bind monomeric actin. Actin (1.0 μm) was incubated with 10 μm GST-Crn1 or GST-N-WASp-VCA bound to glutathione-Sepharose beads or with beads alone (control) before pelleting. B, copelleting assays showing the effect of increasing concentrations of Crn1 on sedimentation of Arp2/3 complex with actin filaments. Arp2/3 complex at 0.5 μm and 0–4 μm Crn1 were incubated with 2.0 μm polymerized actin. Pelleted coronin and Arp2/3 complex were quantified by densitometry. The dashed line with open squares indicates the amount of Crn1 pelleted with F-actin at each concentration. C, copelleting assay showing binding of Crn1 to actin filaments. Phalloidin-stabilized actin filaments were incubated with 40 nm His-Crn1 and pelleted, and supernatant and pellet were blotted with an anti-His antibody. sup., supernatant. D, binding isotherm from actin filament copelleting assays showing the effect of increasing concentrations (conc) of actin filaments on Crn1 sedimentation. Actin filaments were incubated with Crn1-WDU (0.5 μm) or Crn1-UCC (3.0 μm) before pelleting. Data were fit as described under “Experimental Procedures.” E, binding isotherm for Crn1 and Crn1-WD-CC as described in C. F, tabulated binding affinities of Crn1 constructs for actin filaments. G, copelleting assays showing the effect of increasing concentrations of Crn1-ALEE-AKK on sedimentation of Arp2/3 complex with actin filaments assays. Conditions were identical to B. Plots shown in panels B, D, E, and G are representative of at least three separate experiments.