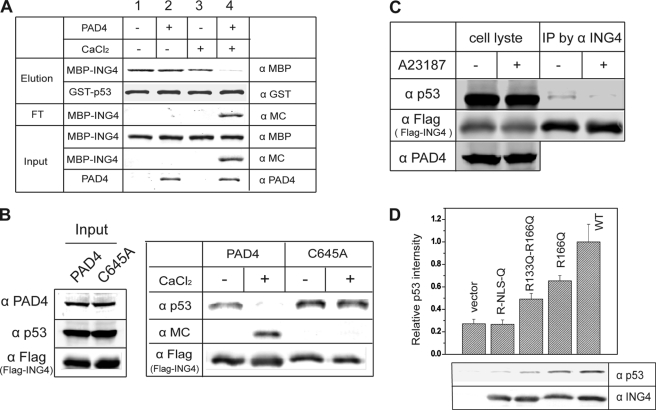
FIGURE 4.
Citrullination of ING4 disrupts p53 binding. A, ING4 citrullination disrupts p53 binding in vitro. Pretreated with combinations of PAD4 and CaCl2 as indicated at top, MBP-ING4 was incubated with resin-bound GST-p53. The input, flow-through (FT), and eluted samples were analyzed by Western blots using anti-PAD4 antibody (α PAD4), anti-modified citrulline (α MC) to detect the citrulline content of ING4, anti-MBP (α MBP) to detect MBP-ING4, and anti-GST antibody (α GST) to detect GST-p53. B, citrullination of ING4 disrupts p53 binding in HEK 293T cell lysates. FLAG-ING4 together with p53 and PAD4 was immunoprecipitated from 293T cells coexpressing FLAG-ING4 and PAD4. Half of the resulting resin was treated with CaCl2 and washed, and the retained proteins were eluted and analyzed by Western blots using anti-modified citrulline, anti-FLAG antibody (α FLAG) to detect FLAG-ING4 and anti-p53 antibody (α p53). C, loss of ING4-p53 interaction in cells with activated PAD4 is shown. HEK 293T cells coexpressing PAD4 and FLAG-ING4 were treated with or without calcium ionophore A23187. FLAG-ING4 was immunoprecipitated (IP), and the coimmunoprecipitated endogenous p53 was detected by anti-p53. Anti-FLAG (α FLAG) was also probed to indicate equal loading of FLAG-ING4. D, citrulline-mimicking mutants of ING4 disrupt binding to p53. FLAG-ING4 and single (R166Q), double (R133Q/R166Q), and tetra (Arg-NLS-Gln) Arg to Gln mutants were generated and expressed in HEK 293T cells. The FLAG epitope immunoprecipitates were analyzed for endogenous p53 binding by Western blot. The ratio of band densities for p53 (α p53) and FLAG-ING4 (α FLAG) are shown and represent three independent experiments.