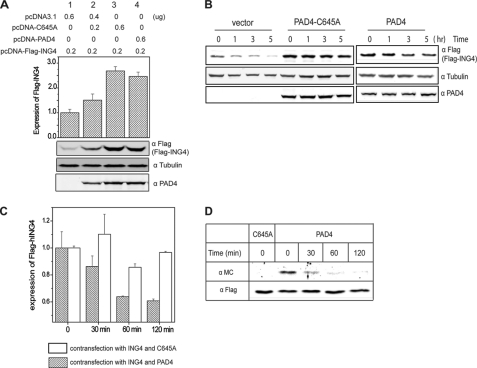
FIGURE 6.
Inactive PAD4 stabilizes ING4 and citrullination of ING4 promotes its degradation in HEK 293T cells. A, cells expressing FLAG-ING4 and wild-type or C645A PAD4 (transfected at two concentrations, shown above) were analyzed by Western blot using anti-FLAG (FLAG-ING4), anti- tubulin (α tubulin), and anti-PAD4 (α PAD4). Relative band densities for FLAG-ING4 and tubulin were determined as in Fig. 5B and are shown at the top. B, inactive PAD4 slows degradation of ING4 in cells. Cells expressing FLAG-ING4 and C645A PAD4, empty vector, or wild-type PAD4 in the absence of a calcium ionophore, were treated with cycloheximide and harvested at later time points for Western blot using anti-FLAG (FLAG-ING4), anti-tubulin, and anti- PAD4. C, active PAD4 promotes ING4 degradation in cells. Cells expressing FLAG-ING4 and wild-type or C645A PAD4 were treated with 5 μm A23187, 2 mm CaCl2, and 100 μg/ml cycloheximide. Cells were harvested at later time points, and the expression of FLAG-ING4 was examined using Western blot as described in B. Data represent three independent experiments and are relative values based on the density at time zero. D, citrullinated ING4 was preferentially degraded. Cells expressing FLAG-ING4 and wild type or C645A PAD4 were treated with 5 μm A23187 in Locke's solution at 37 °C for 30 min and then changed to fresh medium containing 100 μg/ml cycloheximide. Cells were harvested at later time points, and FLAG-ING4 was immunoprecipitated. Citrullination content of ING4 was detected by anti-modified citrulline (α MC). α FLAG was also probed as the loading control.