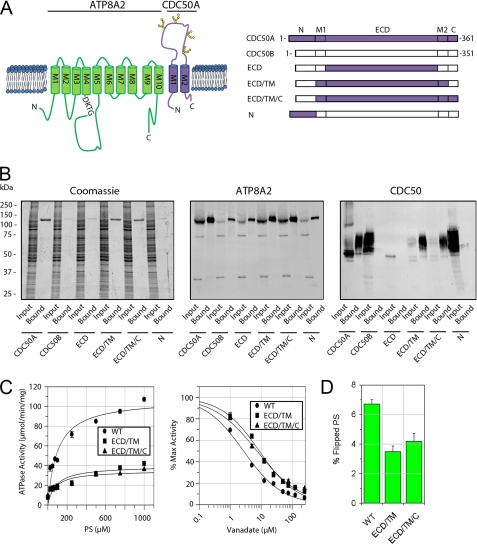
FIGURE 5.
Interaction and functional activity of ATP8A2-CDC50 complexes containing CDC50A/B chimera proteins. A, topological model of the ATP8A2-CDC50A complex (left panel). ATP8A2 is shown in green, and CDC50A is in purple. The phosphorylated motif (DKTG) is indicated along with the positions of the N-linked glycosylation sites on CDC50A. Right panel, schematic showing various chimera CDC50A/B proteins used in these studies. B, interaction of CDC50A/B chimera proteins containing a 1D4 tag with ATP8A2. Proteins were purified on an ATP8A2 immunoaffinity column and analyzed on SDS gels stained with Coomassie Blue or Western blots labeled with an ATP8A2 antibody (ATP8A2) or Rho-1D4 antibody (CDC50). CDC50A co-purified with ATP8A2, whereas CDC50B did not. Chimera proteins containing only the extracellular domain (ECD) or N-terminal domain (N) of CDC50A did not co-purify with ATP8A2. Chimeras containing the ECD and TM domain consisting of both M1 and M2 segments (ECD/TM) or the ECD, TM, and C-terminal domains (ECD/TM/C) of CDC50A co-purified with ATP8A2. C, left panel, ATPase activity of ATP8A2 associated with either WT CDC50A (WT) or chimera CDC50A proteins (ECD/TM or ECD/TM/C) as a function of PS concentration in the presence of PC. Right panel, vanadate inhibition of ATPase activity of ATP8A2-CDC50 complexes in 1000 μm PS in the presence of PC. D, flippase activity of ATP8A2-CDC50 complexes containing WT CDC50A and the ECD/TM and ECD/TM/C chimeras reconstituted in phosphatidylcholine in the presence of 2.5% NBD-PS.