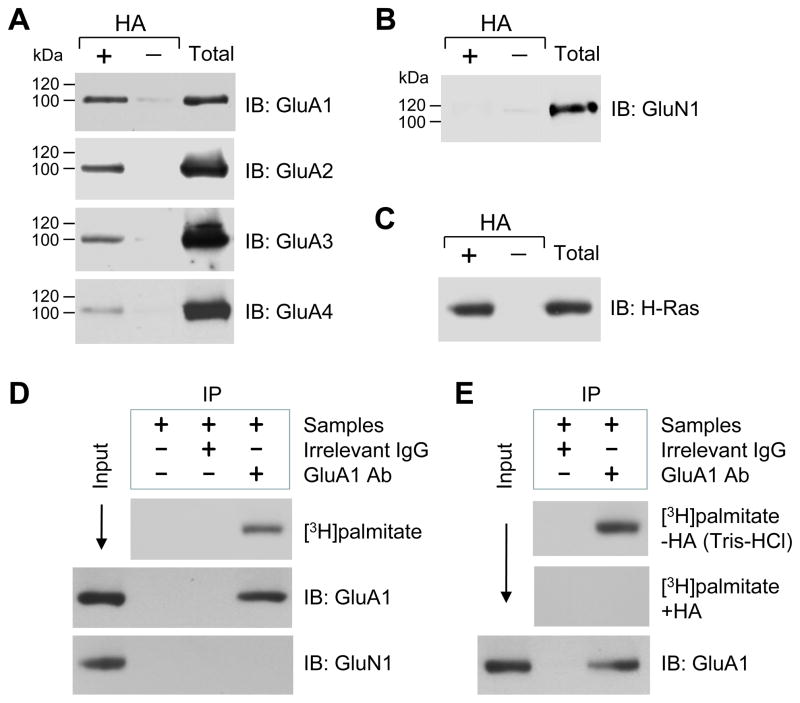
Figure 1. Palmitoylation of native AMPAR subunits GluA1-4 in rat NAc neurons in vivo and in vitro.
(A) Palmitoylation of GluA1-4 subunits in the NAc. (B) Palmitoylation of NMDA receptor GluN1 subunits in the NAc. (C) Palmitoylation of H-Ras in the NAc. Palmitoylation was examined in homogenates from the normal rat NAc tissue using the ABE assay (see Materials and Methods). Proteins that were purified through the ABE method, both in the presence (+) and absence ( ) of HA, were analyzed by immunoblot (IB) using the indicated antibodies. HA-dependent detection of a given protein indicates its palmitoylation. As a control, total proteins from homogenates (before ABE purification) were also examined at the same blot. (D) Palmitoylation of GluA1 as detected in cultured rat NAc neurons by [3H]palmitate metabolic labeling. (E) HA-dependent palmitoylation of GluA1 in cultured NAc neurons. GluA1 proteins were purified via immunoprecipitation (IP), followed by film detection of incorporation of [3H]palmitate into GluA1 and immunoblot confirmation of immunoprecipitated GluA1.