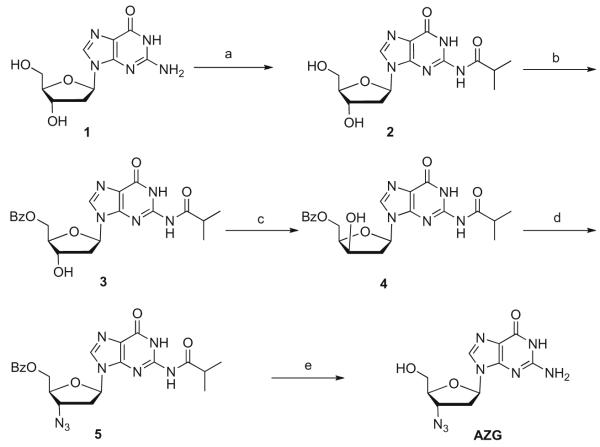
Scheme 1.
Reagents and conditions: (a) TMSCl, (i-BuCO)2O, pyridine, rt, 4 h, 75%; (b) Bz2O, DMF, Et3N, rt 2 h, 74%; (c) (i) Tf2O, DCM, pyridine, 0 °C, (ii) H2O, (iii) MeOH, NaHCO3; 32%; (d) (i) MsCl, DCM, DMAP, 0 °C, 40 min, (ii) NaN3, DMF, 120 °C, 2 h, 65%, two steps; (e) NaOCH3/MeOH, DCM 45 °C, 4 h, 82%.