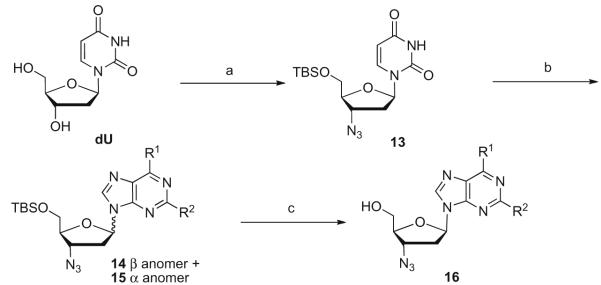
Scheme 4.
Reagents and condition: (a) (i) TBSCl, imidazole, DMF, rt, overnight, 80%, (ii) DIAD, TPP, 80 °C, 30 min, 75%, (iii) LiN3, DMF, 100 °C, 24 h, 70%; (b) purine base, BSA, TMSOTf, CH3CN, 70 °C, 18 h, 34–61%; (c) (i) TBAF/acetic acid (v/v: 1:0.2), THF, rt, 4–6 h, (ii) anomer separation on silica gel, (iii) nucleophilic reagents in the case of 2- and/or 6-chloro purines in one to three steps in 20–95% yield.