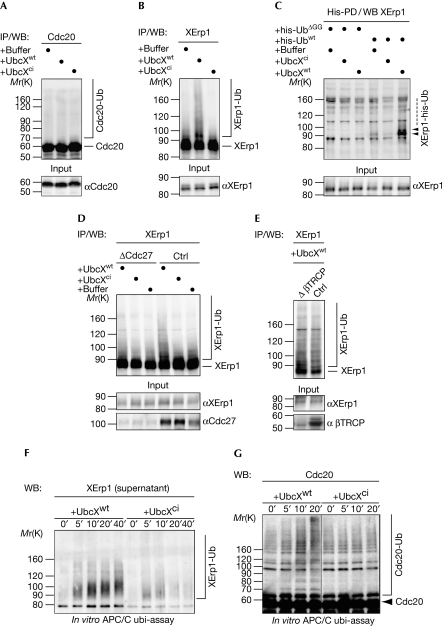
Figure 4.
UbcXwt promotes XErp1 ubiquitylation. (A) CSF extract incubated with buffer, UbcXwt or UbcXci for 30 min was processed for WB analysis (input) or used to IP Cdc20, followed by WB analysis. (B) CSF extract was treated as in (A) and XErp1 was immunoprecipitated and analysed by WB. (C) CSF extracts containing his-Ubwt or his-UbΔGG were treated as in (A) and his-ubiquitin-modified proteins were purified with Ni2+NTA beads under denaturating conditions (his-PD). XErp1 was detected by WB. (D) CSF extract that was depleted of Cdc27 or control-depleted extract was supplemented with the indicated reagent. Samples were taken and Cdc27 and XErp1 were detected by WB analysis (input), or XErp1 was immunoprecipitated and analysed by WB. (E) β-TRCP or control-depleted CSF extract was treated with UbcXwt and processed for WB analysis (input) or used to IP XErp1, followed by WB for XErp1. (F) APC/C was immunoprecipitated from CSF extract using magnetic beads coated with Cdc27 antibodies and treated with UbcXwt or UbcXci for the indicated times. Then, the APC/C was removed from the reaction by the recapture of the beads and XErp1 in the supernatant was detected by WB. (G) APC/C was immunoprecipitated and treated as in (F) and the reactions were analysed by WB for Cdc20 at the indicated timepoints. Note: Samples in A, B, D and E were subjected to CIP phosphatase treatment before WB analysis. Arrowheads indicate modified XErp1. APC/C, anaphase-promoting complex/cyclosome; Cdc, cell division cycle; ci, catalytically inactive; CIP, calf intestinal alkaline phosphatase; CSF, cytostatic factor; his-UbΔGG, his-ubiquitinΔGG; his-Ubwt, his-ubiquitin; IP, immunoprecipitation; PD, pull-down; Ub, ubiquitin; WB, western blotting; wt, wild type; XErp1-Ub, ubiquitin-modified XErp1.