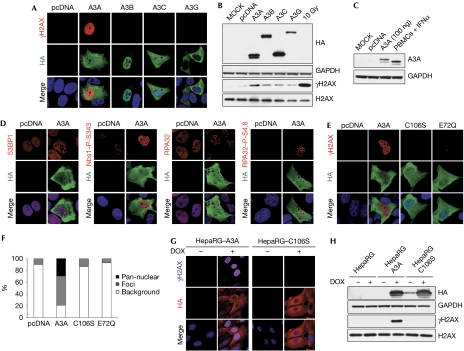
Figure 1.
APOBEC3A expression can induce DNA damage responses. (A) APOBEC3A induces phosphorylation of histone H2AX. U2OS cells left untreated (MOCK) or transfected with plasmids expressing haemagglutinin (HA)-tagged A3A, A3B, A3C and A3G or the pcDNA3.1 control plasmid were fixed 24 h post-transfection, stained with HA and γH2AX antibodies, and analysed by confocal microscopy. (A,D,E) Nuclei were stained with DAPI. Results are representative of three independent experiments. (B) Western blot detection of phosphorylated H2AX in cells transfected with A3A. U2OS cells were transfected with APOBEC3 expression plasmids or pcDNA3.1 as control. Cells were collected at 48 h and lysates were analysed by western blot using HA, H2AX and γH2AX antibodies. Cells irradiated with 10 Gy were used as a positive control. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. Results are representative of three independent experiments. (C) Transfected U2OS cells express A3A at physiological levels. A3A in lysates from U2OS transfected with 100 ng of A3A plasmid was compared with levels of endogenous A3A expressed in human peripheral blood mononuclear cells (PBMCs) stimulated with interferon (IFN)α (1,000 U/ml) for 24 h. Cell lysates were analysed by western blotting using A3A antibodies, and GAPDH was used as a loading control. (D) Activation of DNA repair proteins by A3A. U2OS cells were transfected with an A3A expression vector or pcDNA3.1 as control. After 24 h, cells were fixed and stained with antibodies to 53BP1, phosphorylated Nijmegen breakage syndrome 1 (Nbs1, S343), replication protein A32 (RPA32) and phosphorylated RPA32 (S4,8), and analysed by confocal microscopy. (E) Phosphorylated H2AX is not detected in cells transfected with the inactive A3A mutants C106S and E72Q. U2OS cells were transfected with plasmids expressing wild-type A3A, the C106S or E72Q mutants or the pcDNA3.1 control plasmid. After 24 h, cells were fixed, stained with HA and γH2AX antibodies and analysed by confocal microscopy. (F) Analysis of γH2AX staining in cells transfected with A3A or the C106S and E72Q mutants. Transfected cells were scored for γH2AX staining. The graph shows the percentage of cells with pan-nuclear γH2AX staining, γH2AX foci and background staining. (G,H) Analysis of phosphorylated H2AX in stable HepaRG cells expressing inducible A3A or the C106S mutant. Cells were grown in the presence or absence of doxycycline (1 μg/ml) for 24 h. Fixed cells were analysed by confocal microscopy (G) and cells lysates were analysed by western blot (H). Results are representative of three independent experiments. APOBEC3, apolipoprotein-B mRNA-editing catalytic polypeptide-like 3; DAPI, 4,6-diamidino-2-phenylindole.