Abstract
Studies using Escherichia coli DNA polymerase (Pol) III as the prototype for bacterial DNA replication have suggested that—in contrast to eukaryotes—one replicase performs all of the main functions at the replication fork. However, recent studies have revealed that replication in other bacteria requires two forms of Pol III, one of which seems to extend RNA primers by only a few nucleotides before transferring the product to the other polymerase—an arrangement analogous to that in eukaryotes. Yet another group of bacteria encode a second Pol III (ImuC), which apparently replaces a Pol Y-type polymerase (Pol V) that is required for induced mutagenesis in E. coli. A complete understanding of complex bacterial replicases will allow the simultaneous biochemical screening of all their components and, thus, the identification of new antibacterial compounds.
Keywords: Bacillus subtilis , DNA replication, DNA polymerase III, error-prone polymerase, Pseudomonas aeruginosa
See Glossary for abbreviations used in this article.
Glossary.
- dNTP
deoxyribonucleotide triphosphate
- nt
nucleotide
- Pol
DNA polymerase
- SSB
single-stranded DNA-binding protein
- UV
ultraviolet
Introduction
Cells encode many polymerases that have roles in processes including replication, DNA repair, error avoidance and error fixation. A subset of these polymerases is devoted to most of the chromosomal DNA replication and, together with their accessory proteins, are known as replicases. Cellular chromosomal replicases from all branches of life have three parts (Kornberg & Baker, 1992): a polymerase—Pol III in bacteria, and Pol δ and ε in eukaryotes, a sliding-clamp processivity factor and a clamp loader. Alone, replicative polymerases are not different from other polymerases, but in association with the sliding clamp and clamp loader, they become highly processive (Fay et al, 1981).
Before DNA elongation begins, the Pol III holoenzyme forms an initiation complex through an ATP-dependent reaction (Johanson & McHenry, 1981, 1982; Wickner, 1976; Wickner & Kornberg, 1973). In cells, elongation probably occurs in a tightly coupled series of reactions in which Pol III is chaperoned onto the newly loaded sliding clamp by τ-containing clamp loaders (Downey & McHenry, 2010). Other reviews describe the details of replication by the Escherichia coli Pol III holoenzyme (Bloom, 2009; Johnson & O'Donnell, 2005), which is not discussed here. Bacteria are replicated by family-C DNA polymerases (Pol III holoenzymes), whereas family-B polymerases replicate eukaryotic chromosomes (Braithwaite & Ito, 1993). Although eukaryotic and prokaryotic replicases use almost identical sliding clamps and clamp loaders, there are mechanistic differences between the two systems. In eukaryotes, the clamp loader does not seem to interact with the polymerase, whereas in E. coli it interacts with both Pol III and the replicative helicase, presumably maintaining them in a position in which the replicase can be readily assembled on newly synthesized primers. The E. coli clamp loader binds tightly to Pol III—with a KD of 70 pM (Kim & McHenry, 1996a)—through the carboxy-terminal domain of its τ subunit. As τ is oligomeric, the leading- and lagging-strand polymerases are maintained in one coupled complex (McHenry, 2003). By contrast, Gram-positive clamp loaders bind extremely weakly to their cognate polymerases (Bruck et al, 2005; Bruck & O'Donnell, 2000), suggesting a transient interaction that has greater similarity with the eukaryotic system. However, the τ subunit of Gram-positive bacteria interacts with the replicative helicase (Haroniti et al, 2004)—which is similar to the case in E. coli (Gao & McHenry, 2001; Kim et al, 1996)—and this interaction might increase the concentration of the clamp loader at the replication fork, facilitating τ-subunit interaction with Pol IIIs and coordinating their trafficking at the replication fork.
Another important distinction exists in the number and function of polymerases at the replication fork: in E. coli, the Pol III holoenzyme is the only replicase, whereas there are three replicases in eukaryotes. Pol ε is the eukaryotic leading-strand replicase, Pol δ is the lagging-strand replicase and eukaryotic Pol α is part of the priming apparatus; it elongates nascent primers with dNTPs for a short distance before transferring them to Pol δ (Dua et al, 1999; Nethanel & Kaufmann, 1990; Nick McElhinny et al, 2008; Pursell et al, 2007). In low-GC Gram-positive bacteria, however, two forms of DNA polymerase III are required for chromosomal replication. In addition, other bacteria require a second DNA polymerase III for induced mutagenesis, replacing the error-prone Pol V that was first identified in E. coli.
Hence, the prototype provided by the widely studied E. coli is not universally followed in bacteria. Here, I critically evaluate the emerging knowledge about bacterial chromosomal replication and highlight areas that require further investigation.
Gram-positive bacteria use two forms of DNA polymerase III
Low-GC Gram-positive bacteria have two forms of Pol III: PolC and DnaE (Koonin & Bork, 1996). They are homologous, but PolC has some domains rearranged and an endogenous Mg2+-dependent proofreading activity (Table I). DnaE is more closely related to E. coli Pol III in sequence and domain organization. Genetic and cell physiology studies have indicated that DnaE makes a unique contribution to lagging-strand synthesis in Bacillus subtilis (Dervyn et al, 2001). On the basis of this observation, PolC was proposed to be the leading-strand polymerase and DnaE the lagging-strand polymerase (Dervyn et al, 2001). However, DnaE has low fidelity in vitro (Bruck et al, 2003; Le Chatelier et al, 2004), although its overproduction in vivo does not increase mutation rates (Le Chatelier et al, 2004). These observations argue against a replicative role for this polymerase.
Table 1. Three classes of DNA polymerase III.
| Model organism | Name (or proposed name) | Former names | Function |
|---|---|---|---|
| Escherichia coli | α, DnaE | PolC | Extension of RNA primersRapid, processive chromosomal replication |
| Bacillus subtilis | PolC | DnaF | Rapid, processive chromosomal replication |
| DnaE | Extension of RNA primers | ||
| Pseudomonas aeruginosa | DnaE | Extension of RNA primersRapid, processive chromosomal replication | |
| ImuC | DnaE2 | Error-prone replication, induced mutagenesis |
We reconstituted a rolling circle replication system, using 13 purified B. subtilis replication proteins (Sanders et al, 2010) that had been predicted to be required by previous genetic or biochemical investigations (Bruand et al, 1995, 2001; Bruck & O'Donnell, 2000; Dervyn et al, 2001; Polard et al, 2002; Velten et al, 2003). This system seems to mimic accurately the reaction at the replication fork of a Gram-positive bacterium, in terms of both its correspondence with genetic requirements and the replication-fork rate in vivo (500 nt/s at 30 °C; Wang et al, 2007). Leading-strand replication requires 11 proteins, including the Pol III encoded by polC. The second Pol III encoded by dnaE cannot substitute its function. In addition to these 11 proteins, lagging-strand replication requires DnaE and primase (Sanders et al, 2010), which is consistent with the proposed lagging-strand role for DnaE (Dervyn et al, 2001). However, the elongation rate of DnaE is too slow (approximately 25 nt/s) to keep up with the replication fork. By contrast, PolC supports a physiologically relevant elongation rate (approximately 500 nt/s). PolC discriminates against RNA primers; DnaE uses RNA primers efficiently (Sanders et al, 2010). These characteristics suggest a role for B. subtilis DnaE analogous to that of eukaryotic Pol α, which extends RNA primers and transfers them to a replicase.
The reinterpretation of the structure of a Gram-positive PolC might reveal the mechanism by which it discriminates against an RNA primer. Primer-template binding by PolC differs from DnaE and uses two β-strands of the Thumb domain to interact with the minor groove of the template–primer duplex (Evans et al, 2008). The wider diameter of the A form RNA–DNA duplex does not seem to fit well into the primer-template binding channel of PolC. Thus, an RNA–DNA duplex might not bind strongly, or the conformational changes that are coupled to primer-template binding might not occur completely, leading to improper formation of the catalytic site. These predictions should be tested experimentally.
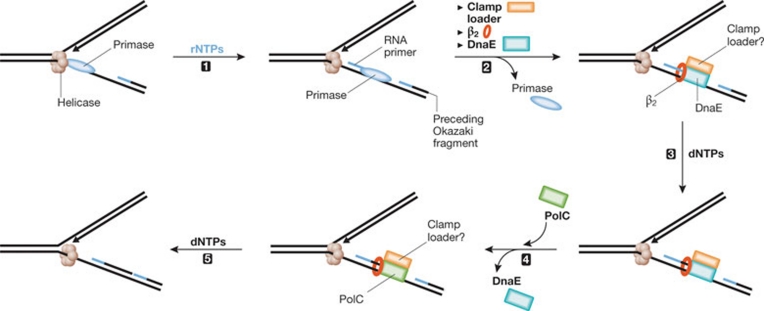
Consistent with the role of Pol α in eukaryotes, RNA-primed single-stranded DNA is used inefficiently by PolC, whereas the addition of low levels of DnaE stimulates a higher level of synthesis than that obtained with DnaE alone (Sanders et al, 2010). However, in the absence of PolC, DnaE can catalyse extensive synthesis, which makes it difficult to determine the position at which the extended primer is transfered from DnaE to PolC. This issue was addressed by using a specific PolC inhibitor (HB-EMAU) developed by George Wright, Neal Brown and colleagues (Tarantino et al, 1999). This class of inhibitor probably acts as a dGTP analogue, forming a ternary complex with template–primer and PolC and trapping the enzyme in a dead-end complex (Low et al, 1974). When HB-EMAU was included in cooperative RNA primer extension reactions containing DnaE and PolC, synthesis was markedly inhibited. This suggests that the transfer to PolC occurs early in the reaction (Sanders et al, 2010), although the precise timing remains to be elucidated. Preliminary experiments suggest that the processivity of DnaE is low, but increases to more than the length of an Okazaki fragment in the presence of B. subtilis SSB, the sliding clamp and known components of the B. subtilis clamp loader (τ, δ, δ′; J.C. Zinder & C.S. McHenry, unpublished data). This suggests that the transfer of the nascent product from DnaE to PolC is probably active, rather than the result of passive ‘falling off’ of DnaE and replacement by PolC (Fig 1). The transfer mechanism might be conceptually similar to that of error-prone polymerase exchange at the replication fork.
Figure 1.
Model for polymerase transfer at the replication fork of low-GC Gram-positive organisms. Primase is brought to the replication fork through interaction with the replicative helicase and (1) synthesizes a short RNA primer that is (2) transferred to DnaE with the assistance of the clamp loader and β2. It is unclear whether the clamp loader remains part of the resulting initiation and elongation complexes. If present, it is probably stabilized by interacting with helicase (Haroniti et al, 2004). (3) The RNA primer is extended by a few nucleotides by DnaE and then (4) transferred to PolC, which (5) catalyses extensive DNA replication and completion of an Okazaki fragment. The molecular interactions that are responsible for DnaE–PolC exchange are unknown. dNTP, deoxyribonucleotide triphosphate; rNTP, ribonucleotide triphosphate.
Benkovic and colleagues have observed dynamic processivity in the T4 system, in which one replicative polymerase can replace another without interrupting elongation (Yang et al, 2004). This polymerase replacement—discovered using a catalytically inactive mutant—requires that the invading polymerase interact with the T4 sliding-clamp protein gp45. In E. coli, Pol II, Pol IV and Pol V can invade the elongating Pol III holoenzyme and gain access to the primer terminus under appropriate conditions (Friedberg et al, 2005; Patel et al, 2010). A tool-belt model was initially proposed, in which multiple polymerases might bind to multiple sites in an oligomeric sliding-clamp processivity factor and switch out at the replication fork (Pages & Fuchs, 2002). However, the interactions of polymerases with sliding clamps are weak when the polymerase cannot access the primer terminus to acquire binding energy, and the off-rates are fast (Kim & McHenry, 1996b). Therefore, if the sliding clamp (β2) is the initial contact with an exogenous polymerase (which remains to be demonstrated), other interactions and contacts must be present to drive, and perhaps regulate, the exchange process. Sutton and colleagues have provided evidence that would limit polymerase interaction to one set of adjacent sites on one half of the β2 sliding clamp (Heltzel et al, 2009), further decreasing the likelihood of a reservoir of polymerases stably associated with β2. In addition to the known E. coli Pol IV–β2 interaction, other contacts between Pol IV and a component of E. coli Pol III* (Pol III + τ-containing clamp loader) trigger Pol III* release (Furukohri et al, 2008). Future research in this area should address the role of the clamp loader in the polymerase-exchange mechanism. It is probably involved in this process, given its role in chaperoning a polymerase onto a newly loaded β2 (Downey & McHenry, 2010) and its possible role in escorting Pol III away from DNA during lagging-strand cycling (P.R. Dohrmann & C.S. McHenry, unpublished data).
Many bacteria can use a mutagenic Pol III
In E. coli, a specialized class of Pol Y polymerases produces induced mutagenesis and stress-induced adaptive modifications (Foster, 2005; McKenzie & Rosenberg, 2001; Pages & Fuchs, 2002; Tippin et al, 2004; Walker, 2005). From the sequencing of many bacterial genomes, it has become apparent that bacteria belonging to several phyla encode two forms of E. coli-like dnaE (not including the PolC–DnaE combinations). The second of these apparently replaces Pol V, the polymerase mainly responsible for induced mutagenesis in E. coli. These two forms of Pol III have sometimes been designated DnaE1 and DnaE2. Two early elegant studies in Mycobacterium tuberculosis and Caulobacter crescentus (Boshoff et al, 2003; Galhardo et al, 2005) associated dnaE2 with a role in induced mutagenesis. In M. tuberculosis, dnaE2 knockout resulted in loss of the enhancement of mutation that accompanies UV irradiation (Boshoff et al, 2003), although overproduction of DnaE2 did not result in mutagenesis, a characteristic of single-subunit Pol Y polymerases in other organisms (Boshoff et al, 2003; Kim et al, 1997). In C. crescentus, dnaE2 knockout reduced the stimulation of mutagenesis following UV irradiation or mitomycin C treatment (Galhardo et al, 2005). Furthermore, DNA polymerase IV (DinB) was not induced by UV irradiation or treatment with mitomycin C, analogous to the situation in M. tuberculosis. In C. crescentus, dnaE2 is the distal gene in an operon preceded by a small gene (imuA) that has weak similarity to E. coli sulA and recA, and a gene similar to Pol Y-like genes (imuB). Knockouts of imuA or imuB ablate induced mutagenesis and are epistatic to dnaE2. Knockout of the Pol IV structural gene dinB does not result in a diminution in UV or mitomycin-C-induced mutation (Galhardo et al, 2005). M. tuberculosis has ImuA and ImuB homologues that are required for DnaE2 function (Warner et al, 2010); I therefore propose to name DnaE2 as ImuC, for clarity (Table I). This is a logical extension of the nomenclature of Menck and co-workers (Galhardo et al, 2005) and will help to distinguish this polymerase from DnaEs that coexist with PolCs, with which they are often confused in the literature.
In the most advanced studies so far, M. tuberculosis ImuB has been shown to interact with the replicative DnaE, ImuC and ImuA in a yeast two-hybrid assay (Warner et al, 2010). ImuB, despite being homologous to Pol Y error-prone polymerases, does not contain the triad of conserved Pol III catalytic acidic residues (Warner et al, 2010) and must therefore be inactive. Mutation of the predicted catalytic aspartic acid residues of ImuC ablates induced mutagenesis (Warner et al, 2010). Thus, ImuC is the error-prone polymerase in M. tuberculosis and presumably other organisms that contain ImuA/B/C and lack Pol V homologues. However, ImuB interacts with β2, but ImuC does not. Thus, it seems that ImuB has an important regulatory role, interacting with β2, the replicative DnaE and the error-prone polymerase ImuC.
What could be the role of an inactive Pol Y polymerase? A mechanism must exist to give Pol Y polymerase access to the replication fork, in order to replace the normal cellular replicase when blocking lesions are encountered. Perhaps ImuB preserves these functions and ImuC catalyses translesion replication. Such a system would be useful to study the non-catalytic activities of Pol Y polymerases. A eukaryotic translesion polymerase with limited activity, Rev1, interacts with other translesion polymerases including Pol κ, Pol ζ, Pol ι and Pol η (Acharya et al, 2005; Guo et al, 2003; Ohashi et al, 2004; Tissier et al, 2004). This could be a functional parallel to the ImuB–ImuC interaction.
In Pseudomonas aeruginosa, an imuC gene is preceded by SulA/RecA-like imuA and Pol Y-like imuB structural genes in an apparent operon. A LexA binding site is found immediately upstream from this operon, suggesting that it is regulated by the SOS response. Mutation of ImuC abolishes UV-induced mutagenesis (Sanders et al, 2006) and knocking out imuB also abolishes mutagenesis (Sidebar A; R.C. Pope, J.C. Lindow, P.R. Dohrmann & C.S. McHenry, unpublished data). These results are in agreement with the M. tuberculosis and C. crescentus systems, but in contrast to a report about another pseudomonad (Koorits et al, 2007). ImuA, B and C are induced by agents that also induce the SOS response and by ciprofloxacin treatment (Blazquez et al, 2006; Brazas & Hancock, 2005; Cirz et al, 2006). No studies have addressed the role of ImuA in ImuC-dependent induced mutagenesis, but given its homology to RecA, it might be involved in the protein–protein interactions that form the nucleoprotein ‘mutasome complex’, as described for the E. coli Pol V-dependent induced mutagenesis pathway (Jiang et al, 2009).
Sidebar A | ImuC might be a therapeutic target to decrease the frequency of antibiotic resistance.
In a seminal study, cystic fibrosis patients were shown to be colonized early in life with a few Pseudomonas aeruginosa strains that remain for the lifetime of the patient and adapt to the environment by mutation (Oliver et al, 2000), a factor that contributes to drug resistance and treatment failure. These strains become progressively hypermutable, often due to mutation of mismatch repair genes as the disease progresses (Ciofu et al, 2005; Hassett et al, 2010; Hogardt et al, 2007; Oliver et al, 2000). In Escherichia coli, induction of SOS mutagenesis has been shown to be important for the evolution of drug resistance, even in the presence of mismatch repair deficiencies (Cirz & Romesberg, 2006). In Mycobacterium tuberculosis, persistence and evolution of drug resistance in animal models is diminished in ImuC knockouts (Boshoff et al, 2003). As the development of drug resistance affects treatment outcome in patients with cystic fibrosis, the ImuC system therefore represents a target for chemotherapy (Smith & Romesberg, 2007).
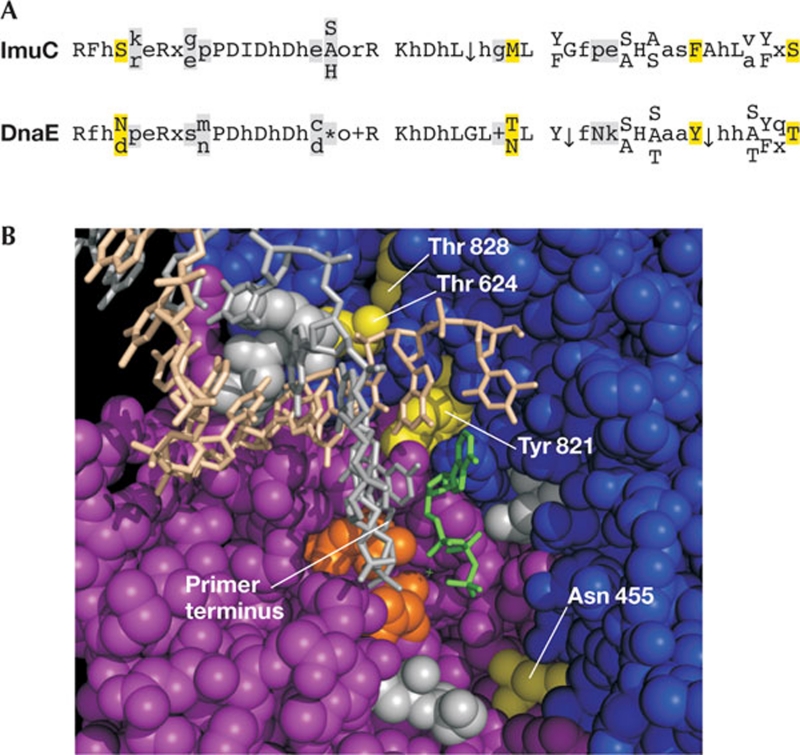
There has been confusion about the relationship between DnaE-like polymerases that coexist in PolC-containing strains and those that coexist with replicative DnaEs. To distinguish between them, I compared the consensus sequences resulting from a comprehensive ImuC alignment with an alignment of DnaEs that exist alone or with PolCs. The latter are similar, and distinguishable from ImuC. There are only a few instances of absolutely conserved residues in ImuC that differ from DnaE, but several elements are absent in ImuC, such as an obvious β2-binding loop (Warner et al, 2010; Fig 2). These differences are near the three catalytic aspartic acids and at the C-terminal end of the Fingers domain. Mapping the differences onto the DNA-dNTP-Taq Pol III α structure shows that these residues line the active site and the end of the DNA-binding channel near the primer terminus. These changes could relax substrate binding in a way that diminishes fidelity and allows bypass, which would be consistent with the error-prone function attributed to ImuC. However, this hypothesis needs to be tested experimentally.
Figure 2.
Conserved residues in ImuC that differ from other DnaE polymerases. (A) Residues conserved in ImuC that differ from DnaE are highlighted in yellow. Other differences are shown in grey. The first two sequence blocks show residues surrounding the three catalytic aspartic acids (PDID and hDh) in the ImuC line. The third block shows sequences at the carboxy-terminal end of the Fingers domain. (B) The active site of Taq Pol III α. Residues that differ from those conserved in ImuC are shown in grey or yellow, as in (A). The three catalytic aspartic acid residues are shown in orange. The incoming dNTP is green, and the primer and template strands are shown in stick form in grey and beige, respectively. The figure was prepared in PyMOL using Protein Data Bank 3E0D. Only the Palm and Fingers domains are shown. Gly 666, Arg 767 and Lys 771 are hidden for clarity. dNTP, deoxyribonucleotide triphosphate; h, hydrophobic amino acid; o, polar amino acid; asterisk, basic amino acid; x, no consensus; arrow, small amino acid.
Chemical biology of DNA replication
DNA replication is an essential process for the proliferation of all pathogens and an unexplored target for the development of new antibacterials. Therapeutically useful inhibitors have been developed that inhibit processes and molecules upstream and dowsntream from DNA replication, such as nucleotide precursor biosynthesis (Hawser et al, 2006) and DNA gyrase (Mitscher, 2005), respectively. Most of the subunits of the bacterial DNA replication apparatus are essential, suggesting that their inhibition should lead to a block in cell proliferation or death (Kornberg & Baker, 1992). This has been shown by a class of compounds, 6-anilinouracils, that are targeted to the polymerase subunit of the Gram-positive replicase PolC. These compounds are not only potent biochemical inhibitors, but also specific blockers of DNA replication in Gram-positive bacteria (Daly et al, 2000). Although screens targeting individual replicase subunits have been described (Butler & Wright, 2008; Georgescu et al, 2008; Shapiro et al, 2005; Yang et al, 2002), complete bacterial replicases have only recently been explored by chemical genetic approaches (Dallmann et al, 2010).
The ten subunits of E. coli DNA Pol III holoenzyme interact to form a complex protein machine (Jeruzalmi et al, 2001; Kim & McHenry, 1996b; Kim et al, 1996; McHenry, 2003; Williams et al, 2003). Protein interactions change at the various steps of the replicative reaction. Taking into account all of the individual protein components and their interactions with other subunits and substrates, it has been estimated that more than 100 essential targets are potentially useful for the development of antibacterial agents (Dallmann et al, 2010). Given the impracticality of running 100 specific screening assays, a biochemical high-throughput screen was developed in which inhibition of any of the essential targets could be detected through a common endpoint (Dallmann et al, 2010). In a trial screen with a small (20,000-compound) library against full replication systems derived from model Gram-negative and Gram-positive organisms, it was possible to distinguish compounds that inhibited the replicase of a single species from compounds that exhibited broad-spectrum potential. Counterscreens against non-orthologous enzymes with related activities identified the compounds that are most likely to be target-specific (Dallmann et al, 2010).
Bacteriophages express inhibitory peptides against several cellular processes, including DNA replication. This is another source of in vitro inhibitors that enable mechanistic studies and allow the identification of potential therapeutic targets. For example, some Staphylococcus aureus phages produce peptides that bind to and inhibit the β2 sliding clamp and DnaI helicase loader (Belley et al, 2006; Liu et al, 2004), and coliphage N4 produces a peptide inhibitor of the E. coli clamp loader that interacts with the δ subunit (Yano & Rothman-Denes, 2011). The crystallization of complexes of such peptides and their targets should provide data that could support library design and the development of small-molecule inhibitors.
Further developments in this area will hopefully provide compounds that facilitate biochemical investigation. Those that specifically inhibit Gram-positive DnaE or P. aeruginosa ImuC will be useful tools to distinguish the cellular and biochemical functions that are different to those of the main replicative Pol III. Stage-specific inhibitors will probably be useful to stop reactions, so that transient intermediates can be isolated and studied, as is the case with inhibitors of stages of nucleotide biosynthesis (Santi & McHenry, 1972; Sintchak et al, 1996) and enzymes involved in other aspects of nucleic acid metabolism (Classen et al, 2003; Ho et al, 2009). Such compounds should be useful in vivo—in combination with classical genetics—for example, to identify resistant mutants for target identification or verification. Chemical genetics provides a route to extend standard genetics. For example, the addition of a compound gives an immediate response, allowing the measurement of the effects of the kinetics of a block, which can also be rapidly reversed. Broad-spectrum compounds can be used to modulate responses in many cell types, including genetically intractable ones.
Conclusions
For decades, E. coli has been the main prototype for bacterial DNA replication, through which the central principles of DNA replication have been established. However, several key studies have now shown that there are important differences between E. coli and other bacteria in DNA replication. Among them are bacteria that use two forms of Pol III for chromosomal replication, subdividing the tasks performed by one E. coli Pol III holoenzyme. In B. subtilis and other low-GC Gram-positive bacteria, PolC, together with a sliding clamp and clamp loader, is the main replicase. However, it is unable to extend the RNA primers for Okazaki fragment synthesis on the lagging strand. A second Pol III, DnaE, assumes the primer extension role and transfers the extended primer to PolC. This reaction is reminiscent of lagging-strand replication in eukaryotes, in which an RNA primer extended by eukaryotic Pol α is handed off to Pol δ. Thus, a reaction that was previously thought to be ‘eukaryotic’ occurs in an important class of bacteria, which include important human pathogens.
In diverse classes of bacteria, the prototypical Pol V that is required for induced mutagenesis in E. coli is replaced by a new Pol III, ImuC, which functions with ImuB, a homologue of standard Pol Y family polymerases that lacks a competent polymerase site. This system also requires a regulated mechanism for the transfer of a primer terminus between two Pol IIIs. Defining the molecular interactions and mechanism for polymerase-transfer reactions is an important challenge in the enzymology of several important processes (Sidebar B). Progress has been hampered, in part, by the inefficiency of the transfer mechanisms previously studied. In B. subtilis, transfer must occur rapidly (in less than 1 s) during the synthesis of each Okazaki fragment. Thus, it might constitute a useful system in which to study this important problem and, perhaps, reveal a universally shared mechanism.
Sidebar B | In need of answers.
What are the interactions between and relative roles of PolC and DnaE in low-GC Gram-positive organisms?
How does PolC transfer extended lagging-strand primers from DnaE?
What additional replicase components exist in Gram-positive organisms, beyond those identified so far by homology searches and genetics?
What is the mechanism used by ImuA, ImuB and the Pol III-like ImuC to catalyse error-prone replication and generate mutations?
The availability of well-defined biochemical assays that include all proteins required for complex replicative transactions has provided an opportunity to discover small-molecule inhibitors that block conformational changes in proteins, macromolecular interactions or freeze complexes at specific reaction stages. These will provide important tools for studying DNA-replicative reactions. A subset of these compounds might lead to the development of antibacterials that specifically block DNA replication and associated processes.
Charles S McHenry
Acknowledgments
I thank Diane Hager for preparation of the figures and assembly of the bibliography and Melissa Stauffer, PhD, of Scientific Editing Solutions, for editing the manuscript. Our work with B. subtilis was supported by US National Science Foundation grant MCB-0919961.
Footnotes
The author declares that he has no conflict of interest.
References
- Acharya N, Haracska L, Johnson RE, Unk I, Prakash S, Prakash L (2005) Complex formation of yeast Rev1 and Rev7 proteins: a novel role for the polymerase-associated domain. Mol Cell Biol 25: 9734–9740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belley A et al. (2006) Competition of bacteriophage polypeptides with native replicase proteins for binding to the DNA sliding clamp reveals a novel mechanism for DNA replication arrest in Staphylococcus aureus. Mol Microbiol 62: 1132–1143 [DOI] [PubMed] [Google Scholar]
- Blazquez J, Gomez-Gomez JM, Oliver A, Juan C, Kapur V, Martin S (2006) PBP3 inhibition elicits adaptive responses in Pseudomonas aeruginosa. Mol Microbiol 62: 84–99 [DOI] [PubMed] [Google Scholar]
- Bloom LB (2009) Loading clamps for DNA replication and repair. DNA Repair (Amst) 8: 570–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshoff HI, Reed MB, Barry CE, Mizrahi V (2003) DnaE2 polymerase contributes to in vivo survival and the emergence of drug resistance in Mycobacterium tuberculosis. Cell 113: 183–193 [DOI] [PubMed] [Google Scholar]
- Braithwaite DK, Ito J (1993) Compilation, alignment, and phylogenetic relationships of DNA polymerases. Nucleic Acids Res 21: 787–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazas MD, Hancock RE (2005) Ciprofloxacin induction of a susceptibility determinant in Pseudomonas aeruginosa. Antimicrob Agents Chemother 49: 3222–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruand C, Ehrlich SD, Janniere L (1995) Primosome assembly site in Bacillus Subtilis. EMBO J 14: 2642–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruand C, Farache M, McGovern S, Ehrlich SD, Polard P (2001) DnaB, DnaD and DnaI proteins are components of the Bacillus subtilis replication restart primosome. Mol Microbiol 42: 245–256 [DOI] [PubMed] [Google Scholar]
- Bruck I, O'Donnell ME (2000) The DNA replication machine of a Gram-positive organism. J Biol Chem 275: 28971–28983 [DOI] [PubMed] [Google Scholar]
- Bruck I, Goodman MF, O'Donnell ME (2003) The essential C family DnaE polymerase is error-prone and efficient at lesion bypass. J Biol Chem 278: 44361–44368 [DOI] [PubMed] [Google Scholar]
- Bruck I, Georgescu RE, O'Donnell M (2005) Conserved interactions in the Staphylococcus aureus DNA polC chromosome replication machine. J Biol Chem 280: 18152–18162 [DOI] [PubMed] [Google Scholar]
- Butler MM, Wright GE (2008) A method to assay inhibitors of DNA polymerase IIIC activity. Methods Mol Med 142: 25–36 [DOI] [PubMed] [Google Scholar]
- Ciofu O, Riis B, Pressler T, Poulsen HE, Hoiby N (2005) Occurrence of hypermutable Pseudomonas aeruginosa in cystic fibrosis patients is associated with the oxidative stress caused by chronic lung inflammation. Antimicrob Agents Chemother 49: 2276–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirz RT, Romesberg FE (2006) Induction and inhibition of ciprofloxacin resistance-conferring mutations in hypermutator bacteria. Antimicrob Agents Chemother 50: 220–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirz RT, O'Neill BM, Hammond JA, Head SR, Romesberg FE (2006) Defining the Pseudomonas aeruginosa SOS response and its role in the global response to the antibiotic ciprofloxacin. J Bacteriol 188: 7101–7110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen S, Olland S, Berger JM (2003) Structure of the topoisomerase II ATPase region and its mechanism of inhibition by the chemotherapeutic agent ICRF-187. Proc Natl Acad Sci USA 100: 10629–10634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallmann HG et al. (2010) Parallel multiplicative target screening against divergent bacterial replicases: Identification of specific inhibitors with broad spectrum potential. Biochemistry 49: 2551–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly JS, Giehl TJ, Brown NC, Zhi C, Wright GE, Ellison RT III (2000) In vitro antimicrobial activities of novel anilinouracils which selectively inhibit DNA polymerase III of Gram-positive bacteria. Antimicrob Agents Chemother 44: 2217–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dervyn E, Suski C, Daniel R, Bruand C, Chapuis J, Errington J, Janniere L, Ehrlich SD (2001) Two essential DNA polymerases at the bacterial replication fork. Science 294: 1716–1719 [DOI] [PubMed] [Google Scholar]
- Downey CD, McHenry CS (2010) Chaperoning of a replicative polymerase onto a newly-assembled DNA-bound sliding clamp by the clamp loader. Mol Cell 37: 481–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dua R, Levy DL, Campbell JL (1999) Analysis of the essential functions of the C-terminal protein/protein interaction domain of Saccharomyces cerevisiae pol ε and its unexpected ability to support growth in the absence of the DNA polymerase domain. J Biol Chem 274: 22283–22288 [DOI] [PubMed] [Google Scholar]
- Evans RJ, Davies DR, Bullard JM, Christensen J, Green LS, Guiles JW, Pata JD, Ribble WK, Janjic N, Jarvis TC (2008) Structure of polC reveals unique DNA binding and fidelity determinants. Proc Natl Acad Sci USA 105: 20695–20700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay PJ, Johanson KO, McHenry CS, Bambara RA (1981) Size classes of products synthesized processively by DNA polymerase III and DNA polymerase III holoenzyme of Escherichia coli. J Biol Chem 256: 976–983 [PubMed] [Google Scholar]
- Foster PL (2005) Stress responses and genetic variation in bacteria. Mutat Res 569: 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg EC, Lehmann AR, Fuchs RP (2005) Trading places: how do DNA polymerases switch during translesion DNA synthesis? Mol Cell 18: 499–505 [DOI] [PubMed] [Google Scholar]
- Furukohri A, Goodman MF, Maki H (2008) A dynamic polymerase exchange with Escherichia coli polymerase IV replacing polymerase III on the sliding clamp. J Biol Chem 283: 11260–11269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galhardo RS, Rocha RP, Marques MV, Menck CF (2005) An SOS-regulated operon involved in damage-inducible mutagenesis in Caulobacter crescentus. Nucleic Acids Res 33: 2603–2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, McHenry CS (2001) τ binds and organizes Escherichia coli replication proteins through distinct domains. Domain IV, located within the unique C terminus of τ, binds the replication fork helicase, DnaB. J Biol Chem 276: 4441–4446 [DOI] [PubMed] [Google Scholar]
- Georgescu RE, Yurieva O, Kim SS, Kuriyan J, Kong XP, O'Donnell M (2008) Structure of a small-molecule inhibitor of a DNA polymerase sliding clamp. Proc Natl Acad Sci USA 105: 11116–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Fischhaber PL, Luk-Paszyc MJ, Masuda Y, Zhou J, Kamiya K, Kisker C, Friedberg EC (2003) Mouse Rev1 protein interacts with multiple DNA polymerases involved in translesion DNA synthesis. EMBO J 22: 6621–6630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroniti A, Anderson C, Doddridge Z, Gardiner L, Roberts CJ, Allen S, Soultanas P (2004) The Clamp-loader-helicase interaction in Bacillus. Atomic force microscopy reveals the structural organisation of the DnaB−τ complex in Bacillus. J Mol Biol 336: 381–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett DJ, Korfhagen TR, Irvin RT, Schurr MJ, Sauer K, Lau GW, Sutton MD, Yu H, Hoiby N (2010) Pseudomonas aeruginosa biofilm infections in cystic fibrosis: insights into pathogenic processes and treatment strategies. Expert Opin Ther Targets 14: 117–130 [DOI] [PubMed] [Google Scholar]
- Hawser S, Lociuro S, Islam K (2006) Dihydrofolate reductase inhibitors as antibacterial agents. Biochem Pharmacol 71: 941–948 [DOI] [PubMed] [Google Scholar]
- Heltzel JM, Maul RW, Scouten Ponticelli SK, Sutton MD (2009) A model for DNA polymerase switching involving a single cleft and the rim of the sliding clamp. Proc Natl Acad Sci USA 106: 12664–12669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MX, Hudson BP, Das K, Arnold E, Ebright RH (2009) Structures of RNA polymerase–antibiotic complexes. Curr Opin Struct Biol 19: 715–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogardt M, Hoboth C, Schmoldt S, Henke C, Bader L, Heesemann J (2007) Stage-specific adaptation of hypermutable Pseudomonas aeruginosa isolates during chronic pulmonary infection in patients with cystic fibrosis. J Infect Dis 195: 70–80 [DOI] [PubMed] [Google Scholar]
- Jeruzalmi D, O'Donnell ME, Kuriyan J (2001) Crystal structure of the processivity clamp loader gamma complex of E. coli DNA polymerase III. Cell 106: 429–441 [DOI] [PubMed] [Google Scholar]
- Jiang Q, Karata K, Woodgate R, Cox MM, Goodman MF (2009) The active form of DNA polymerase V is UmuD'(2)C-RecA-ATP. Nature 460: 359–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson KO, McHenry CS (1981) Role of the β subunit of the Escherichia coli DNA polymerase III holoenzyme in the initiation of DNA elongation. In Structure and DNA–Protein Interactions of Replication Origins, Ray D (ed), pp 425–436. New York, USA: Academic [Google Scholar]
- Johanson KO, McHenry CS (1982) The β subunit of the DNA polymerase III holoenzyme becomes inaccessible to antibody after formation of an initiation complex with primed DNA. J Biol Chem 257: 12310–12315 [PubMed] [Google Scholar]
- Johnson A, O'Donnell M (2005) Cellular DNA replicases: components and dynamics at the replication fork. Annu Rev Biochem 74: 283–315 [DOI] [PubMed] [Google Scholar]
- Kim DR, McHenry CS (1996a) Biotin tagging deletion analysis of domain limits involved in protein-macromolecular interactions: Mapping the τ binding domain of the DNA polymerase III α subunit. J Biol Chem 271: 20690–20698 [DOI] [PubMed] [Google Scholar]
- Kim DR, McHenry CS (1996b) Identification of the β-binding domain of the α subunit of Escherichia coli polymerase III holoenzyme. J Biol Chem 271: 20699–20704 [DOI] [PubMed] [Google Scholar]
- Kim S, Dallmann HG, McHenry CS, Marians KJ (1996) Coupling of a replicative polymerase and helicase: a τ–DnaB interaction mediates rapid replication fork movement. Cell 84: 643–650 [DOI] [PubMed] [Google Scholar]
- Kim SR, Maenhaut-Michel G, Yamada M, Yamamoto Y, Matsui K, Sofuni T, Nohmi T, Ohmori H (1997) Multiple pathways for SOS-induced mutagenesis in Escherichia coli: an overexpression of dinB/dinP results in strongly enhancing mutagenesis in the absence of any exogenous treatment to damage DNA. Proc Natl Acad Sci USA 94: 13792–13797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin EV, Bork P (1996) Ancient duplication of DNA polymerase inferred from analysis of complete bacterial genomes. Trends Biochem Sci 21: 128–129 [PubMed] [Google Scholar]
- Koorits L, Tegova R, Tark M, Tarassova K, Tover A, Kivisaar M (2007) Study of involvement of ImuB and DnaE2 in stationary-phase mutagenesis in Pseudomonas putida. DNA Repair (Amst) 6: 863–868 [DOI] [PubMed] [Google Scholar]
- Kornberg A, Baker TA (1992) DNA Replication. New York, USA: WH Freeman Company [Google Scholar]
- Le Chatelier E, Becherel OJ, D'Alencon E, Canceill D, Ehrlich SD, Fuchs RP, Janniere L (2004) Involvement of DnaE, the second replicative DNA polymerase from Bacillus subtilis, in DNA mutagenesis. J Biol Chem 279: 1757–1767 [DOI] [PubMed] [Google Scholar]
- Liu J et al. (2004) Antimicrobial drug discovery through bacteriophage genomics. Nat Biotechnol 22: 185–191 [DOI] [PubMed] [Google Scholar]
- Low RL, Rashbaum SA, Cozzarelli NR (1974) Mechanism of inhibition of Bacillus subtilis DNA polymerase III by the arylhydrazinopyrimidine antimicrobial agents. Proc Natl Acad Sci USA 71: 2973–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry CS (2003) Chromosomal replicases as asymmetric dimers: studies of subunit arrangement and functional consequences. Mol Microbiol 49: 1157–1165 [DOI] [PubMed] [Google Scholar]
- McKenzie GJ, Rosenberg SM (2001) Adaptive mutations, mutator DNA polymerases and genetic change strategies of pathogens. Curr Opin Microbiol 4: 586–594 [DOI] [PubMed] [Google Scholar]
- Mitscher LA (2005) Bacterial topoisomerase inhibitors: quinolone and pyridone antibacterial agents. Chem Rev 105: 559–592 [DOI] [PubMed] [Google Scholar]
- Nethanel T, Kaufmann G (1990) Two DNA polymerases may be required for synthesis of the lagging DNA strand of simian virus 40. J Virol 64: 5912–5918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick McElhinny SA, Gordenin DA, Stith CM, Burgers PMJ, Kunkel TA (2008) Division of labor at the eukaryotic replication fork. Mol Cell 30: 137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi E, Murakumo Y, Kanjo N, Akagi J, Masutani C, Hanaoka F, Ohmori H (2004) Interaction of hREV1 with three human Y-family DNA polymerases. Genes Cells 9: 523–531 [DOI] [PubMed] [Google Scholar]
- Oliver A, Cantón R, Campo P, Baquero F, Blázquez J (2000) High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 288: 1251–1254 [DOI] [PubMed] [Google Scholar]
- Pages V, Fuchs RPP (2002) How DNA lesions are turned into mutations within cells? Oncogene 21: 8957–8966 [DOI] [PubMed] [Google Scholar]
- Patel M, Jiang Q, Woodgate R, Cox MM, Goodman MF (2010) A new model for SOS-induced mutagenesis: how RecA protein activates DNA polymerase V. Crit Rev Biochem Mol Biol 45: 171–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polard P, Marsin S, McGovern S, Velten M, Wigley DB, Ehrlich SD, Bruand C (2002) Restart of DNA replication in Gram-positive bacteria: functional characterisation of the Bacillus subtilis PriA initiator. Nucleic Acids Res 30: 1593–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pursell ZF, Isoz I, Lundstrom EB, Johansson E, Kunkel TA (2007) Yeast DNA polymerase ε participates in leading-strand DNA replication. Science 317: 127–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders GM, Dallmann HG, McHenry CS (2010) Reconstitution of the B. subtilis replisome with 13 proteins including two distinct replicases. Mol Cell 37: 273–281 [DOI] [PubMed] [Google Scholar]
- Sanders LH, Rockel A, Lu H, Wozniak DJ, Sutton MD (2006) Role of Pseudomonas aeruginosa din B-encoded DNA polymerase IV in mutagenesis. J Bacteriol 188: 8573–8585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi DV, McHenry CS (1972) 5-Fluoro-2′-deoxyuridylate: covalent complex with thymidylate synthetase. Proc Natl Acad Sci USA 69: 1855–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A, Rivin O, Gao N, Hajec L (2005) A homogeneous, high-throughput fluorescence resonance energy transfer-based DNA polymerase assay. Anal Biochem 347: 254–261 [DOI] [PubMed] [Google Scholar]
- Sintchak MD, Fleming MA, Futer O, Raybuck SA, Chambers SP, Caron PR, Murcko MA, Wilson KP (1996) Structure and mechanism of inosine monophosphate dehydrogenase in complex with the immunosuppressant mycophenolic acid. Cell 85: 921–930 [DOI] [PubMed] [Google Scholar]
- Smith PA, Romesberg FE (2007) Combating bacteria and drug resistance by inhibiting mechanisms of persistence and adaptation. Nat Chem Biol 3: 549–556 [DOI] [PubMed] [Google Scholar]
- Tarantino PM, Zhi C, Gambino JJ, Wright GE, Brown NC (1999) 6-Anilinouracil-based inhibitors of Bacillus subtilis DNA polymerase III: antipolymerase and antimicrobial structure–activity relationships based on substitution at uracil N3. J Med Chem 42: 2035–2040 [DOI] [PubMed] [Google Scholar]
- Tippin B, Pham P, Goodman MF (2004) Error-prone replication for better or worse. Trends Microbiol 12: 288–295 [DOI] [PubMed] [Google Scholar]
- Tissier A, Kannouche P, Reck MP, Lehmann AR, Fuchs RP, Cordonnier A (2004) Co-localization in replication foci and interaction of human Y-family members, DNA polymerase polη and REVl protein. DNA Repair (Amst) 3: 1503–1514 [DOI] [PubMed] [Google Scholar]
- Velten M, McGovern S, Marsin S, Ehrlich SD, Noirot P, Polard P (2003) A two-protein strategy for the functional loading of a cellular replicative DNA helicase. Mol Cell 11: 1009–1020 [DOI] [PubMed] [Google Scholar]
- Walker GC (2005) Lighting torches in the DNA repair field: development of key concepts. Mutat Res 577: 14–23 [DOI] [PubMed] [Google Scholar]
- Wang JD, Sanders GM, Grossman AD (2007) Nutritional control of elongation of DNA replication by (p)ppGpp. Cell 128: 865–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner DF, Ndwandwe DE, Abrahams GL, Kana BD, Machowski EE, Venclovas C, Mizrahi V (2010) Essential roles for imuA'- and imuB-encoded accessory factors in DnaE2-dependent mutagenesis in Mycobacterium tuberculosis. Proc Natl Acad Sci USA 107: 13093–13098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S (1976) Mechanism of DNA elongation catalyzed by Escherichia coli DNA polymerase III, dnaZ protein and DNA elongation factors I and III. Proc Natl Acad Sci USA 73: 3511–3515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W, Kornberg A (1973) DNA polymerase III star requires ATP to start synthesis on a primed DNA. Proc Natl Acad Sci USA 70: 3679–3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CR, Snyder AK, Kuzmic P, O'Donnell ME, Bloom LB (2003) Mechanism of loading the Escherichia coli DNA polymerase III sliding clamp I: two distinct activities for individual ATP sites in the γ complex. J Biol Chem 279: 4376–4385 [DOI] [PubMed] [Google Scholar]
- Yang F, Dicker IB, Kurilla MG, Pompliano DL (2002) PolC-type polymerase III of Streptococcus pyogenes and its use in screening for chemical inhibitors. Anal Biochem 304: 110–116 [DOI] [PubMed] [Google Scholar]
- Yang J, Zhuang Z, Roccasecca RM, Trakselis MA, Benkovic SJ (2004) The dynamic processivity of the T4 DNA polymerase during replication. Proc Natl Acad Sci USA 101: 8289–8294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano ST, Rothman-Denes LB (2011) A phage-encoded inhibitor of Escherichia coli DNA replication targets the DNA polymerase clamp loader. Mol Microbiol 79: 1325–1338 [DOI] [PubMed] [Google Scholar]