Fig. (1). A schematic representation of various pathological states of tau originating from normal brain tau and associated loss of normal and gain of toxic functions.
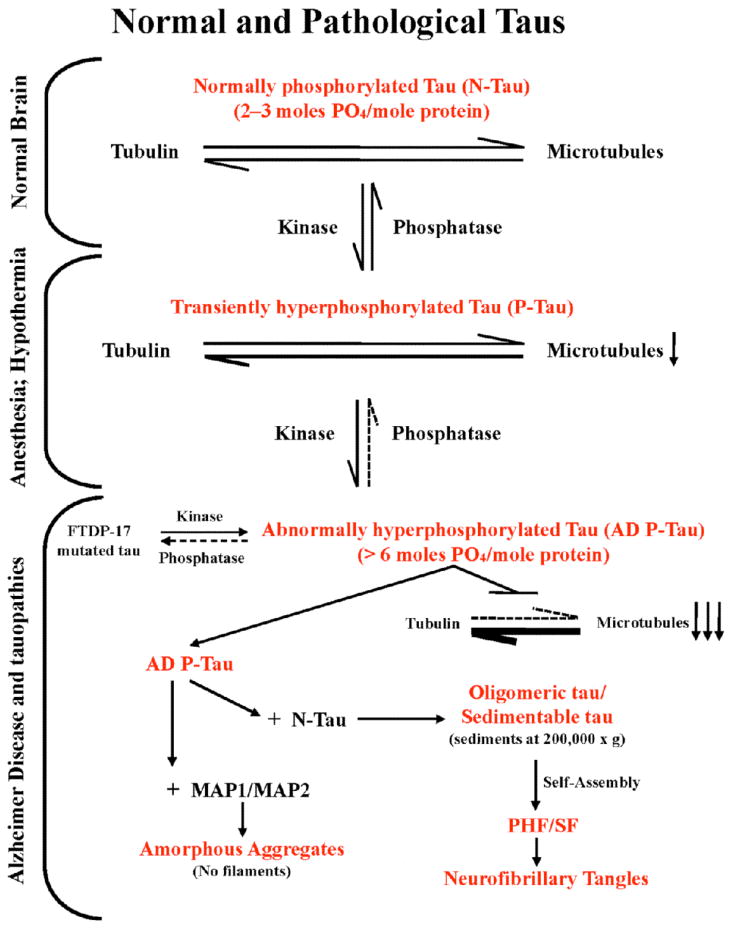
Normal brain tau, which has a stoichiometry of 2–3 moles phosphate/mole of the protein, stimulates assembly of tubulin and stabilizes the structure of microtubules produced. During development, anesthesia as well as hypothermia, such as during hibernation, tau is transiently hyperphosphorylated. During development the level of brain tubulin is >4 mg/ml, the critical concentration required for its self-assembly into microtubules and the role of tau for this function is less critical. The hyperphosphorylation of tau during anesthesia and hypothermia, however, leads to a decrease in microtubule network and the associated functions.
In AD brain a phosphorylation/dephosphorylation imbalance caused apparently by a decrease in protein phosphatase-2A activity leads to abnormal hyperphosphorylation of tau. This AD P-tau on one hand sequesters normal MAPs from microtubules and causes inhibition and disruption of microtubules. On the other hand, while the binding of AD P-tau to MAP1 or MAP2 results in amorphous aggregates, the binding to normal tau forms oligomers. Unlike normal tau, which is highly soluble, the tau oligomers formed with AD P-tau can be sedimented at 200,000 × g and self-assemble into PHF/SF in the form of neurofibrillary tangles.
