Summary
Objective
To determine the prevalence of female genital schistosomiasis in riparian communities in the Volta basin of Ghana,
Design
The study was a cross-sectional study conducted among women 15–49 years in the Volta Basin. Urinary schistosomiasis prevalence was determined using microscopy. A structured questionnaire was also administered to collect information on the demography, obstetric history and reproductive health experiences. Cervical punch biopsy was collected from women who consented to be screened for FGS. Descriptive statistics was used to determine frequency of occurrence, chi squared and logistic regression to identify associated variables
Results
Urinary schistosomiasis prevalence among the women was 24.8% while 10.6% of them diagnosed with FGS. More FGS diagnosed women (57.7%, p value =0.04%) were observed to report copious discharge, vaginal itch (80.8%, p=0.042) and lower abdominal pain (66.7%, p= 0.041) compared to FGS negative women. The predominant abnormal observation of the lower genital tract made was erythematous cervix (18.8%).
Conclusion
The study confirms the reproductive health symptoms associated with FGS and recommends awareness creation on FGS among women in endemic communities to facilitate early treatment.
Keywords: Female Genital Schistosomiasis, lower abdominal pain, vaginal itch, Volta Basin
Introduction
Female Genital Schistosomiasis (FGS) occurs when ova or adults of the trematode parasite, Schistosoma sp ectopically locate in the female genital system.1 Although any of the Schistosoma parasites that affect humans may cause FGS, its occurrence is more common with S. haematobium infections, the causative agent of urinary Schistosomiasis.2–3
FGS prevalence in urinary schistosomiasis endemic areas is estimated to range between 30–50%.1 Even in the absence of ova excretion in women, 23–41% of them are found to have genital schistosomiasis.4–5 FGS has been implicated in women's reproductive health.
The consequences include spontaneous abortions, infertility, postcoital bleeding, dypareunia vaginal discharge, pelvic pain, and genital itch.4,6,7 It has also been shown to facilitate the transmission of sexually transmitted infections such as the human papilloma virus (HPV) and HIV-1through the stimulation of the immune system with a strong bias towards type 2 CD4 helper T cells (Th2).
HIV replication is known to proceeds rapidly in activated T cells thus FGS is considered a risk factor to HIV transmission1. Urinary schistosomiasis is prevalent and widespread in Ghana. However, there is paucity of information of FGS in the country and even worldwide.
Although as high as 40 million women of child bearing age are infected with schistosomiasis and 20 million suffer from the disease, the actual contributions of schistosomiasis to maternal health and birth outcomes for instance are not known.8
Also, schistosomiasis control efforts are mostly targeted at children whilst neglecting other risk groups such as reproductive aged women. To influence schistosomiasis control policy, through evidence based studies, to direct efforts to women in reproductive age in Ghana, this study was conducted to provide baseline data on FGS of the lower reproductive tract in the Volta basin of Ghana and determine how the disease is manifested in infected women.
Methods
Study Area
The study was conducted in fifteen riparian communities on the Afram arm of the Volta River and the Lower Volta basin of Ghana. The women of these communities generally engaged in trading including fish mongering and subsistence farming. Their main water contact activity was collection of water from the Volta Lake for domestic use. Most of the women were also exposed to water contact through water transport as they moved from one community to another.
Study Design
This was a cross-sectional study carried out among women 20–49 years who are permanent residents in riparian communities on the Afram arm of the Volta River and the lower Volta Basin. Women 15–19 years, although considered to be within the reproductive age, were excluded on the grounds of being among the school age group.
However those within this age group who were married and wanted to participate were recruited. Virgins, pregnant women, menstruating women, postnatal women and women who had undergone hysterectomy were excluded from the screening process. Initial visits were made to the villages to explain the details of the study to both community leaders and community members to ensure approval and support to participate in the study.
Meetings were also held with the women in the villages to solicit their opinions and suggestions and address their concerns. Community members were informed of the use of identification numbers instead of names and confidentiality of study findings. Eligibility to participate in the study was determined by gender, informed consent and permanent residency in village. Women who consented to participate were examined by a gynaecologist for their fitness to participate in the study.
Those who were deemed fit by the doctor were administered a structured questionnaire entailing sociodemographic information and reproductive health experiences.
Mid-day urine samples were collected and examined for S. haematobium ova by microscopy. After this cervical biopsy collection was done by the gynaecologist who inspected the vulva and vagina for abnormalities.
With the aid of a sterile disposable vaginal speculum, the appearance of the cervix was also noted, and cervical biopsy was taken from suspicious lesions in the anterior lip of the cervix using a 5mm cervical punch biopsy forceps. The collected tissue was immediately placed on a microscope glass slide. A drop of trypan blue was added to the tissue to facilitate clearing and identification of ova.
The tissue sample was then compressed between two microscope glass slides secured with a tape and placed in a moist chamber at 4°C for transportation to the Noguchi Memorial Institute for Medical Research (NMIMR) laboratory for analysis. To minimize non participation bias, free transport to the screening centres and lunch were provided to participants.
Where district hospitals were over 100 km away from the community and local health facilities were found to be unsuitable (such as bat infested premises) for use, make shift screening centres were set up for the gynaecological screening.
All women diagnosed for FGS were treated with praziquantel within a period of 3 months, and those found with other health conditions during screening were referred through the existing health systems to the nearest appropriate health facility for treatment.
Treatment for urinary schistosomiasis was offered to all infected subjects including those who did not consent to gynaecological screening. Ethical clearance was given by the Institutional Review Board of Noguchi Memorial Institute for Medical Research.
Statistical analysis
The data was entered and analysed using SPSS statistical software, version 16.0. Descriptive statistics were obtained through frequencies and cross tabulations. Comparison between groups was made using Chi- square test and fisher exact tests where appropriate. The analysis was two-tailed and the level of significance set at 5%. Binary logistic regression was also used to assess the effects of confounders where necessary.
Results
Four hundred and twenty (420) subjects consented to undergo gynaecological screening. About ninety three (93.4%) of them were married and (6.6%) in a permanent sexual relationship.
The mean age of the study participants was 33.6 years and the modal age 40 years. Out of the 420 women, 25 were excluded from undergoing screening: 6 menstruating on the day of screening, 2 hysterectomy done, 3 with early pregnancy, 6 not feeling well, 4 husbands refused their wives participation and 4 withdrew consent. Three hundred and ninety five of them were therefore screened gynaecologically and within this cohort 371 of them provided urine.
Schistosomiasis Prevalence
Urine schistosomiasis prevalence determined among the women was 25.3% (Table 1), while FGS prevalence found was 10.6% (42/395). Only 13 (14.1%) of urinary schistosomiasis positive women were diagnosed with FGS and 24 (8.6%) of the urinary schistosomiasis negative women diagnosed for FGS.
Table 1.
Female genital schistosomiasis prevalence among subjects screened for urinary schistosomiasis
| FGS occurrence among study subjects | |||
| Urinary schistosomiasis status |
FGS Positive (%) | FGS Negative (%) | Total (%) |
| Positive | 13(14.1) | 79 (85.9) | 92(24.8) |
| Negative | 24(8.6) | 255 (91.4) | 279(75.2) |
| Total | 37(10.0) | 334(90.0) | 371(100) |
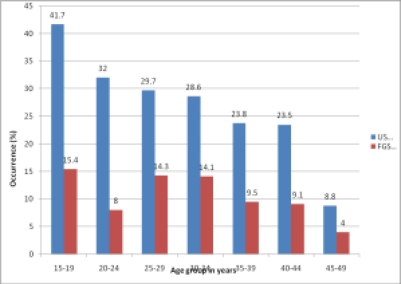
No significant association was observed between the occurrence of urinary schistosomiasis and FGS of the cervix (χ2 =1.79, p value=0.18). While urinary schistosomiasis prevalence decreased with increasing age FGS prevalence remained relatively stable among the various age groups (Figure 1).
Figure 1.
Age distribution of urinary schistosomiasis and female genital schistosomiasis in the study population
Manifestation of Female Genital Schistosomiasis
Vaginal itching (62.3%) and vaginal discharge (56%) were the most frequently reported reproductive health problem. Although no significant difference was observed between women with FGS reporting vaginal discharge and women without FGS (p value =0.55), more FGS diagnosed women (57.7%) were observed to report copious discharge than women without FGS (36.9% )(p value =0.04). Women with FGS also reported more vaginal itch (80.8%, p=0.042) and lower abdominal pain (66.7%, p= 0.041) than FGS negative women.
While number of births a woman has had confounded the effect of FGS on lower abdominal pain (OR=1.8), irregular menstruation (OR=4.6), odorous vaginal discharge (OR=4.9) and dyspareunia (OR=2.2) enhanced lower abdominal pain in the presence of FGS. Post-coital bleeding was, however, not associated with FGS (p > 0.05) (Table 2).
Table 2.
Logistic regression to determine confounders of FGS on Lower Abdominal pain
| Variables | Crude OR (95%CI |
p-value | Adjusted OR (95% CI) |
p-value |
| FGS | 2 (1.01–3.92) | 0.044 | ||
| Number of births |
1.8 (0.93–3.63) | 0.08 | ||
| Irregular menstruation | 2 (1.02–3.95) | 0.043 | ||
| Odorous discharge | 4.9 (1.5–15.01) | 0.005 | ||
| Dyspareunia | 2.2(1.12–4.48) | 0.022 | ||
| Post coital bleeding |
1.8 (0.94–3.68) | 0.07 | ||
FGS associated abnormalities of the lower reproductive tract
The abnormalities of the lower reproductive tract observed in the women have been shown in Table 3.
Table 3.
Clinical observation of external genitalia
| Clinical Observation | No. Positive | % Positive |
| Vulva/Vagina | ||
| Ulcer | 3/398 | 0.8 |
| Oedema | 3/398 | 0.8 |
| Erosion | 8/398 | 2 |
| Discharge | 133/398 | 33.4 |
| Cervix | ||
| Erythematous | 73/389 | 18.8 |
| Punctuate | 6/387 | 1.6 |
| Sandy patches | 12/388 | 3.1 |
| Growths | 12/388 | 3.1 |
Discussion
Ghana is one of the first five countries implementing the integrated control program for Neglected Tropical Diseases (NTD). However due to lack of evidence based data to highlight the reproductive health importance of schistosomiasis, the strategy to reduce disease burden continues to mainly focus on children 9 while neglecting other risk groups including reproductive aged women.
This study provides evidence based data on female genital schistosomiasis in the Volta Basin. The study found 10.6% of women in their reproductive age to be positive for FGS. Although other studies reported higher prevalence, 49% in Zimbabwe 4, 36% in Northern Tanzania 10 and 33.3% in Madagascar 7, the prevalence found in the Volta Basin is still significant to be of public health concern.
Considering that health consequences of FGS include infertility, miscarriages and ectopic pregnancies, and the negative impact of these on families and societies are well known, this study brings out the need to further investigate FGS impact on women's health. For instance, half of women studied in a hamlet in Egypt were found to have FGS and its associated reproductive health morbidity11. However, these women were unwilling to complain about their health situation to their husbands for fear of being divorced or husband taking another wife.
Also, because FGS symptoms are generally not distinguishable from those of sexually transmitted infections (STI) the condition is most often mistaken as an STI, which is mostly stigmatized. A possible reason that may be ascribed to the observed prevalence of FGS in this study may be related to low virulence of the parasite type in this region.12
As early as 1956, it was shown that, the West African strain of S. haematobium produced a milder form of schistosomiasis morbidity and mortality compared to the Egyptian strain of the parasite 12. , Differences in urinary tract pathology associated with Schistosoma infection were reported among various regions of Africa and even within communities.13,14
Other factors such as hosts immunity and environmental influences also have significant effects on schistosomiasis morbidity. Urinary schistosomiasis prevalence followed the standard trend of decreasing with increasing age which was consistent with findings from earlier studies.15
Also as observed by this study, the stable trend of FGS infection among the various age groups indicates that all women in the reproductive age are at the risk of FGS. Some studies have determined the occurrence of sandy patches as pathognomonic for FGS.16,17
However, in this study, gynaecological examination did not include colposcopy, which promotes better 11,16 observation of sandy patches on the cervix.11,16
This explains in part the low observation of sandy patches in this study and the insignificant association observed (p= 0.63) between it and FGS diagnosed by crushed biopsy. The predominant pathologic cervical abnormality observed among the women was erythematous cervix. Other observations were strawberry cervical growths, punctuate cervix and sandy patches.
Generally, women with FGS were seen more with genital lesions than women without schistosomiais (p>0.05). The presence of cervical lesions in association with schistosomiasis has been well reported to be indicative of various health problems including cervical cancer.1,5,6
Vulva lesions in both women and girls have also been documented in case reports of FGS18,19 This occurs when worms migrate from the ano-rectal plexus to the internal pudendal vein (lower part of the sacral plexus) and the posterior labial vein or via the vesical plexus to the santorini plexus via the deep vein of the clitoris.18
Feldmeier and his colleagues2 therefore suggested that, any woman with urinary schistosomiasis may at least or temporarily have Schistosoma lesions in her genitals. This may account for urinary schistosomiasis positive women showing more vulva lesions than uninfected women (p>0.05). Significant symptoms associated with FGS found in this study were copious vaginal discharge, vaginal itch and lower abdominal pain.
This is comparable to the study conducted by Kjetland et al., (2008) who found genital itch, malodorous and abnormally coloured discharge as significant symptoms associated with FGS infection in rural Zimbabwean women 20. Leutcher et al. 7 found pelvic pain, vaginal discharge and irregular menstruation as predictors of FGS. It was however not surprising that number births a woman had confounded the effects of lower abdominal pain. Spontaneous vaginal deliveries (SVD) are known to have a toll on a woman's pelvic region.
Thus in rural communities were successful deliveries are mainly SVDs, the more births a woman went through the more she may be of risk of pelvic related problems.
Contrary to findings from this study, Kjetland et al. 20 reported no gynaecological complaints associated with urinary schistosomiasis infection whereas women with genital schistosomiasis significantly reported more stress incontinence and frequent micturition.
Individuals diagnosed with urinary schistosomiasis in this study reported more dyspareunia (painful sexual intercourse) (p-value=0.03). Admitting that dyspareunia may be common younger women initiating sex and postmenopausal women as a result of decreased oestrogen, other and individuals with sexually transmitted infections, these variables did not confound dyspareunia in urinary schistosomiasis infected women in this study.
Although selection bias may affect the epidemiological validation of these results, these findings nevertheless confirm the importance of schistosomiasis in reproductive health. As the consequences of this condition may be impacting negatively on maternal health, it will be recommended that awareness is created about female genital schistosomiasis in endemic areas.
Acknowledgement
Sincere thanks go to the Ghana AIDS Commission and the Ministry of Water Resources, Works and Housing for funding the study. Also acknowledged are departments of Parasitology and Bacteriology of Noguchi Memorial Institute for Medical Research (NMIMR) for technical support, Public health Unit of the Volta River Authority for technical support and supply of Praziquantel for treatment, The District Health Managements teams of districts involved in study, Community leaders and community members without whose participation the study would have been impossible.
References
- 1.Poggensee G, Feldmeir H, Krantz I. Schistosomiasis of the Female Genital Tract: Public Health Aspects. Parasitology Today. 1999;5(9):378–381. doi: 10.1016/s0169-4758(99)01497-0. [DOI] [PubMed] [Google Scholar]
- 2.Feldmeier H, Daccal RC, Martins MJ, Soares V, Martins R. Genital Manifestations of Schistosoma mansoni in Women: Important but Neglected. Mem Inst Oswaldo Cruz. 1998;93(1):127–133. doi: 10.1590/s0074-02761998000700018. [DOI] [PubMed] [Google Scholar]
- 3.Felmeier H, Poggensee G, Krantz I, Helling-Giese G. Female genital schistosomiasis. New challenges from a gender perspective. Trop Geograph Med. 1995b;47:1–15. [PubMed] [Google Scholar]
- 4.Kjetland EF, Ndhlovu PD, Mduluza Gomo E, Gwanzura L, Mason PR, Kurewa EN, Midzi N, Friis H, Gundersen S. Simple clinical manifestations of genital schistosoma haematobium in Rural Zimbawean women. AmJ TropMedHyg. 2005;72(3):311–319. [PubMed] [Google Scholar]
- 5.Poggensee G, Kiwelu I, Saria M, Richter L, Krantz I, Feldmeir H. Schistosomiasis of the lower reproductive tract without egg excretion in urine. American Journal of Tropical Medicine and Hygiene. 1998;59(5):782–783. doi: 10.4269/ajtmh.1998.59.782. [DOI] [PubMed] [Google Scholar]
- 6.Poggensee G, Sahebali S, Marck VE, Swai B, Krantz I, Felmeir H. Diagnosis of Genital cervical schistosomiais: Comparison of Cytological, Histopathological and Parasitological examination. American Journal of Tropical Medicine and Hygiene. 2001;65(3):233–236. doi: 10.4269/ajtmh.2001.65.233. [DOI] [PubMed] [Google Scholar]
- 7.Leutscher P, Ravaolimalala C, Raharisolo CE, Ramarokoto M, Rasendramino A, Raobelison B, Vennervald P E, Feldmeir H. Clinical findings in female genital schistosomiasis in Madagascar. Tropical Medicine and International Health. 1998;3(4):327–333. doi: 10.1046/j.1365-3156.1998.00230.x. [DOI] [PubMed] [Google Scholar]
- 8.Engels D, Chitsulo L, Montresor A, Savioli L. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Trop. 2002;82:139–146. doi: 10.1016/s0001-706x(02)00045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghana Health Service, author. Two year strategic plan for Integrated Neglected Tropical Diseases Control in Ghana, 2007–2008. Ghana Health Service; 2007. [Google Scholar]
- 10.Poggensee G, Krantz I, Kiwelu I, Feldmeir H. Screening of Tanzania women of childbearing age for urinary schistosomiasis: validity of urine reagent strip readings and self-reported symptoms. Bulletin of World Health Organization. 2000;78(4):542–548. [PMC free article] [PubMed] [Google Scholar]
- 11.Talaat M, Watts S, Mekheimar S, Ali HF, Howaida H. The social context of reproductive health in an Egyptian hamlet: a pilot to identify female genital schistosomiasis. Social Science and Medicine. 2004;58:515–524. doi: 10.1016/j.socscimed.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 12.McCullough F. The susceptibility and resistance of Bulinus (Physopsis) globosus and Bulinus (Bulinus) truncatus rohlfsi to two strains of Schistosoma haematobium in Ghana. Bull World Health Organisation. 1959;20(1):75–85. [PMC free article] [PubMed] [Google Scholar]
- 13.Elem B, Vandal MT. Bilharziasis of the urinary tract in Zambia (observation on 100 consecative cases) Med J Zambia. 1981;15(14):48–51. [PubMed] [Google Scholar]
- 14.Pugh RN, Burrows JW, Tayo MA. Malumfasi endemic diseases research project XIV, Increasing Schistosomiasis transmission 1980. Annal Trop med and Para. 74(5):569–570. doi: 10.1080/00034983.1980.11687387. [DOI] [PubMed] [Google Scholar]
- 15.Fulford AJC, Webster M, Ouma JH, Kimani G, Dunne DW. Puberty and age related changes in susceptibility to schistosome infection. Parasitology Today. 1998;14:23–26. doi: 10.1016/s0169-4758(97)01168-x. [DOI] [PubMed] [Google Scholar]
- 16.Helling-Giese G, Sjaatad A, Poggense G, Kjetland EF, Richter J, Chitsulo L, Kumwenda N, Racz P, Roald B, Gundersen SG, Krantz I, Feldmeier H. Female genital schistosomiasis (FGS): relationship between gynaecological and histopathological findings. Acta Trop. 1996;62:257–267. doi: 10.1016/s0001-706x(96)00027-7. [DOI] [PubMed] [Google Scholar]
- 17.Kjetland EF, Helling-Giese G., XXX Female genital schistosomiasis due to S. Haematobium. Clinical and parasitological findings in women in rural Malawi. Acta Tropica. 1996;62:239–255. doi: 10.1016/s0001-706x(96)00026-5. [DOI] [PubMed] [Google Scholar]
- 18.Laven JSE, Vleugels MPH, Dofferhoff ASM, Bloembergen P. Schistosomiasis haematobium as a cause of vulvar hypertrophy. European Journal of Obstetrics and Gynaecology and Reproductive Biology. 1998;79(2):213–216. doi: 10.1016/s0301-2115(98)00033-5. [DOI] [PubMed] [Google Scholar]
- 19.Goldsmith PC, Leshe TA, Saws v, Bryceson AD, Allason-Jones E, Dowd PW. Lesions of Schistosomiasis mime kinp warts on the vula. BMJ. 1993;307(6903):556–557. doi: 10.1136/bmj.307.6903.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kjetland EF, Ndhlovu PD, Kurewa EN, Midzi N, Gomo E, Mduluza T, Friis H, Gundersen SG. Prevention of Gynaecologic Contact Bleeding and Genital Sandy Patches by Childhood Anti-Schistosomal Treatment. Am J Trop Med Hyg. 2008;79(1):79–83. [PubMed] [Google Scholar]