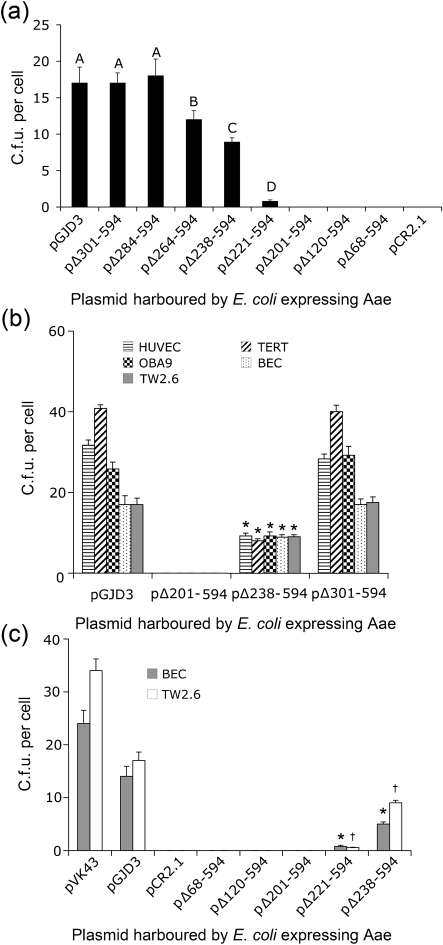
Fig. 2.
Binding of plasmid-harbouring E. coli DH5α cells to freshly isolated human BECs and cultured epithelial cells. (a) Binding of E. coli harbouring the indicated plasmid expressing deletion mutant Aae to BECs. Values show mean c.f.u. per epithelial cell for three independent experiments and error bars indicate sd. Bars with different letters indicate values that were significantly different from each other by ANOVA and post-hoc pairwise testing. (b) Comparison of binding of E. coli DH5α to cells of epithelial origin when expressing Aae and truncated varieties of Aae proteins. BECs, HUVECs, TERT, OBA9 and TW2.6 cells are compared. Similarity between TW2.6 cells and BECs is seen when binding of full-length Aae and truncated versions of Aae protein are compared. Significant differences (P<0.05) for all cell types are seen when full-length Aae is compared to 238–594 aa truncated proteins. * Significantly lower binding to corresponding epithelial cells versus full-length expressed Aae (pGJD3). (c) Direct comparison of binding of E. coli DH5α to BECs and TW2.6 cells when E. coli expresses aae from pVK43 versus pGJD3. Plasmid pVK43 expressing Aae in DH5α binds at a higher level than that seen for pGJD3. DH5α expressing 201–594, 221–594 and 238–594 aa truncated proteins show significant reductions (P<0.05) in binding to their corresponding epithelial cell type (* for BEC, † for TW2.6 cells) when compared to DH5α expressing full-length protein.