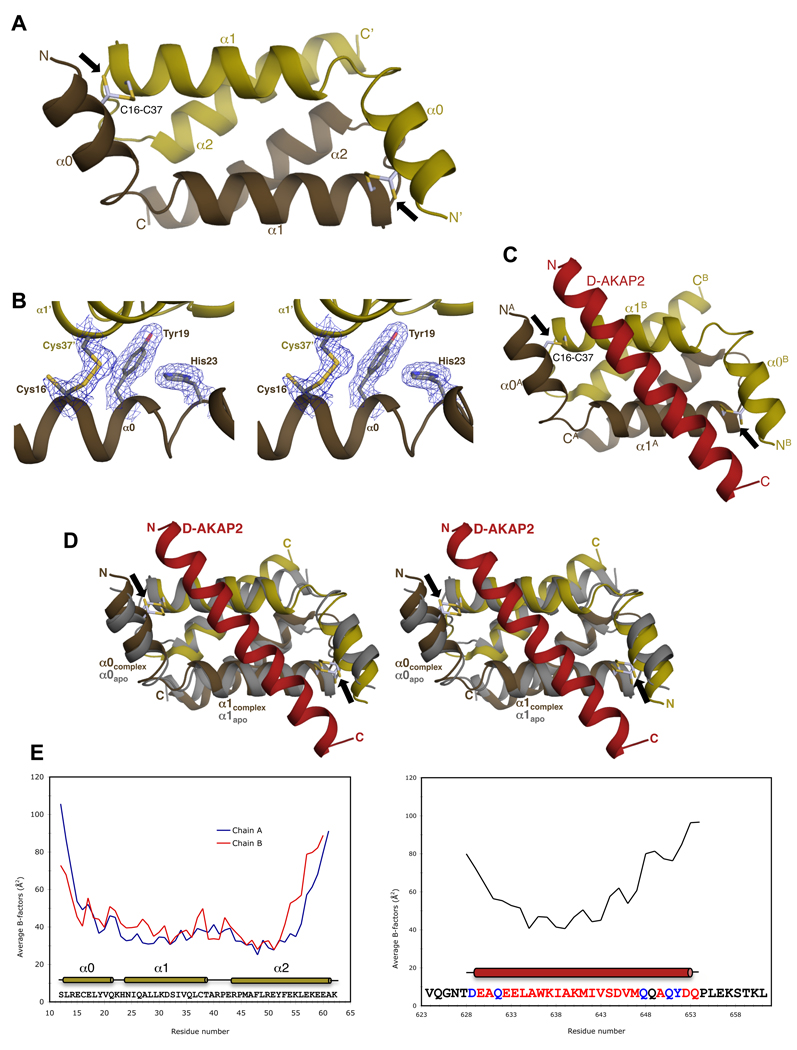
Figure 2. Overview of Apo RIα D/D and RIα D/D:D-AKAP2 structures.
(A) Overall structure of Apo RIα D/D showing the anti-parallel, four-helix bundle. The D/D domain monomer is colored gold with the other monomer, generated by the crystallographic 2-fold symmetry, colored brown. The termini and the helices are labeled. The intermolecular disulfide bonds between residues Cys16 and Cys37 are shown as ball-and-stick models and indicated by a black arrow. (B) Stereoview of the 2Fo–Fc density map contoured at 1 ρrms (root-mean-square electron density of map often reported as σ) shows clear density for the partially reduced disulfide bond and packing residues. The secondary structure elements and the residues are labeled. (C) Overall structure of the RIα D/D domain complexed with the AKB region of D-AKAP2. The monomers of the D/D domain are depicted in gold and brown and D-AKAP2 is shown in red. The locations of the disulfide bonds are indicated by black arrows. (D) Stereoview of overlay of the complex RIα D/D dimer with the apo RIα D/D. RIα D/D:D-AKAP2 structure is colored as Figure 2C and apo RIα D/D is colored gray. Secondary structural elements and the termini are labeled. Black arrows indicate the position of the disulfide bonds. (E) Correlation of crystallographic B-factors with H/D-exchange protection data. The average main chain B-factors of the two monomers of RIα D/D and the AKB region of D-AKAP2 are plotted against residue number. The sequence of the crystallized proteins and the location of the secondary structural elements are indicated below the plot. Residues not modeled or modeled as Ala in the structure are in black and blue respectively. In previous H/D MS experiments, the core of the AKB region showed increased protection from solvent upon binding to the D/D domain consistent with lower B-factors. At the termini, the lack of protection is consistent with the higher B-factors and weak electron density.