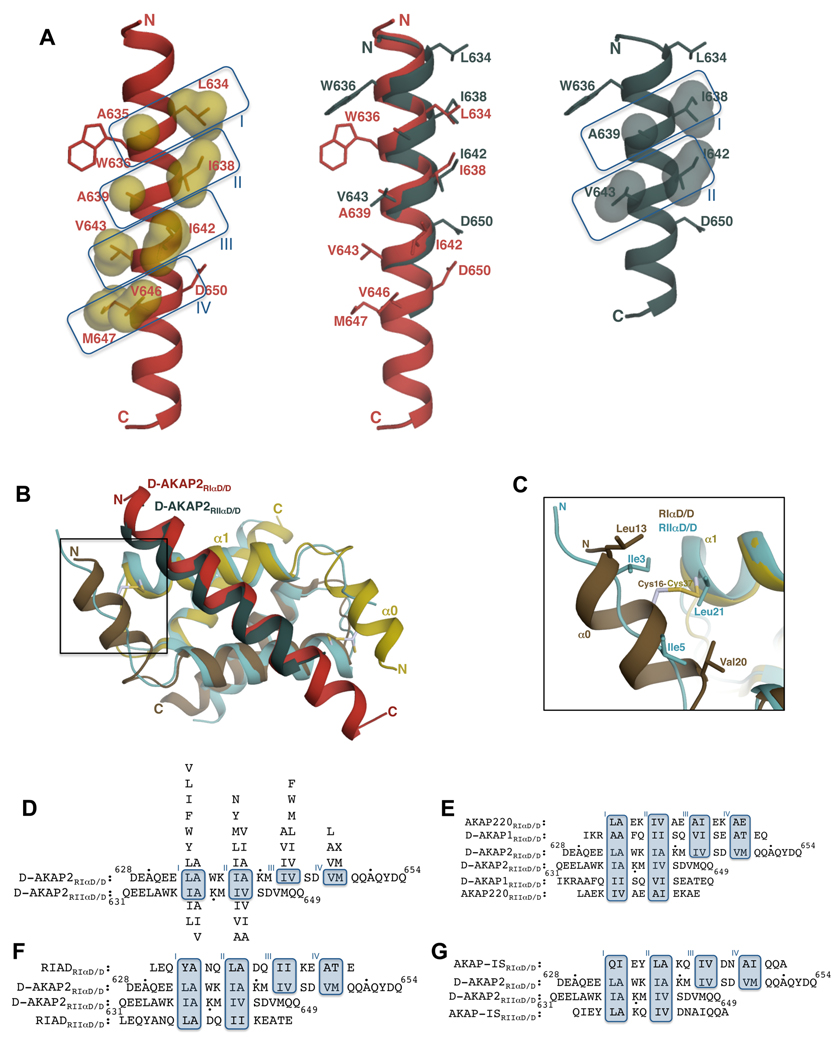
Figure 4. Comparison of RIα D/D:D-AKAP2 and RIIα D/D:D-AKAP2 structures revealing D-AKAP2 specificity.
(A) Structural overlay of D-AKAP2 bound to RIα D/D (in red) and to RIIα D/D (in teal; PDB code: 2HWN) (Kinderman et al., 2006) highlighting the shift in the helical register. Pocket residues are labeled. Residues Trp636 and Asp650 at the termini are also shown to facilitate visualization of the register shift. D-AKAP2 bound to RIα (left) and RIIα (right) are also shown to indicate the pocket positions. Pocket residues are represented as surfaces. (B) Structural overlay of RIα D/D:D-AKAP2 complex with the RIIα D/D:D-AKAP2 complex. The RIα D/D complex is colored as Figure 3A. The D/D domain of RIIα is colored light blue and the D-AKAP2 peptide is colored teal. The helices and the termini are labeled with the respective colors. The N-terminal region that houses the disulfide bond in RIα is boxed. (C) A close-up view of the N-terminal region showing the disulfide bond region of RIα D/D and the equivalent residues from RIIα D/D. For clarity, the D-AKAP2 peptides bound to the two structures are not shown. Cys37 is structurally equivalent to Leu21 of RIIα whereas Cys16 does not have any structurally equivalent counterparts. (D) Structure-based alignment of D-AKAP2 sequence bound to RIα and RIIα. The residue numbers of the N- and C-termini of D-AKAP2 are shown and every tenth residue indicated by a black dot. Due to the shift in the helical register, different residues occupy structurally equivalent pockets of RIα and RIIα. Binding pockets are boxed and labeled. Residues that can replace the pocket residues without disrupting the interaction are shown above (for RIα) and below (for RIIα) the alignment in order of preference. (E–G) Alignment of dual-specific (E), RI-specific (F) and RII-specific (G) AKAPs to the aligned D-AKAP2 sequences according to their expected mode of binding to both RIα and RIIα. Dual-specific AKAPs satisfy the requirements for binding to both R subunits whereas RI- or RII-specific AKAPs satisfy criteria to binding to RI or RII subunits.