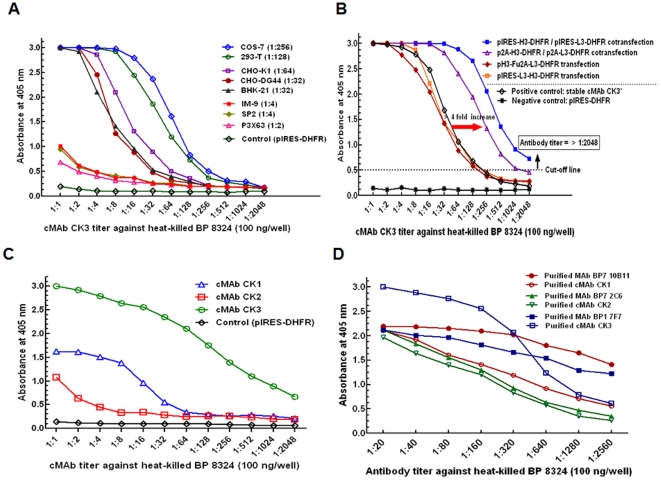
Figure 4. Binding activity of cMAbs produced by mammalian cells with different vector systems.
(A) Selection of suitable host cells for transient production of cMAbs. The cMAb CK3 was transiently produced by 8 different mammalian host cells (∼5×106 cells each/10 cm dish) for 5 days post-transfection using a LF-2000 reagent. The undiluted supernatants including a control (supernatant from CHO-K1 cells transfected by only pIRES-DHFR vector without chimeric chains), were used for cMAb CK3 titration. The number in the parenthesis mark indicates the cMAb CK3 titer. (B) Performance of the re-engineered vector systems for cMAb CK3 production was evaluated by CHO-K1 cells transfected using a LF-LTX reagent. Five days post-transfection of the vectors (10 µg) into the cells (∼1×106 cells/10 cm dish), the undiluted supernatants were used for cMAb CK3 titration, including controls (stable cMAb CK3* at 2 µM MTX: pIRES-H3-DHFR/pIRES-L3-DHFR and culture supernatant by only pIRES-DHFR vector). (C) Binding property of 3 cMAbs produced by CHO-DG44 cells without DHFR amplification. For ELISA, the pIRES-H(L)-DHFR vectors containing chimeric H-chain (H1, H2, and H3) or L-chain (L1, L2, and L3) were cotransfected into CHO-DG44 cells (∼106 cells/10 cm dish) using a LF-2000 reagent and the master seed cells were incubated for 3 days to get sufficient amounts of cMAbs before the DHFR-amplification process. (D) Comparison of binding property of stable cMAbs with the original mouse MAbs. Affinity-purified antibodies were adjusted to the same concentration starting with 5 µg/ml.