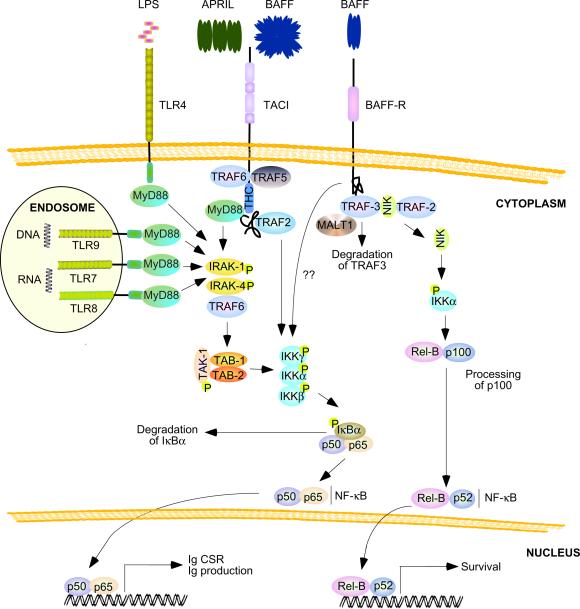
Figure 3.
BAFF and APRIL signaling pathways in B cells. Soluble BAFF multimers or cell-bound APRIL multimers trigger recruitment of TRAF2, TRAF5 and TRAF6 to TACI, thereby activating an IKK complex that mediates phosphorylation of IκBα, followed by proteasomal degradation of IκBα. In the absence of IκBα, p50–p65 and p50c-Rel (not shown), NF-κB dimers translocate from the cytoplasm to the nucleus, where they activate genes involved in Ig CSR and production. A TACI highly conserved (THC) domain also recruits MyD88 similarly to TLR4 (expressed by mouse but not human B cells), TLR7, TLR8 and TLR9, which are receptors for microbial lipolpolysaccharide (LPS), single-stranded RNA and CpG-rich DNA. These TLRs are particularly abundant in B cells and recruit MyD88 through a TIR domain. MyD88 signals from TACI and TLRs activate IKK through IRAK-1, IRAK-4, TRAF6, TAK-1, TAB-1 (TAK-1-binding protein 1), and TAB-2 (TAK-1-binding protein 2). Soluble BAFF trimers induce recruitment of TRAF3 to BAFF-R, followed by TRAF3 degradation through a mechanism involving interaction of TRAF3 with TRAF2 and MALT1. In the absence of TRAF3, NIK elicits phosphorylation of p100, followed by processing of p100 to p52 and translocation of p52-RelB complexes from the cytoplasm to the nucleus, where they activate genes involved in B cell survival. BAFF-R can also activate the canonical NF-κB pathway through a mechanism that remains unclear. CSR, class switch recombination; IkB, inhibitor of NF-κB; IKK, IκB kinase; IRAK, IL-1 receptor-associated kinase; MALT1, mucosa-associated lymphoid tissue lymphoma translocation protein 1; MyD88, myeloid differentiation primary response gene 88; NIK, NF-κB-inducing kinase; TIR doman, Toll-IL-1 receptor domain; TRAF, TNF receptor-associated factor.