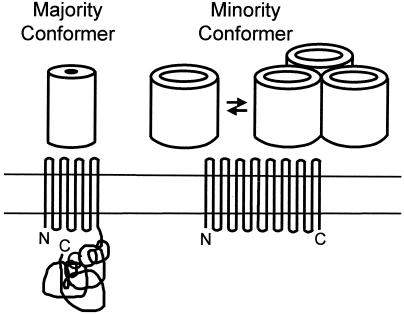
FIG. 3.
Folding model of OmpA-OprF family slow porins. The major fraction of the population folds as a two-domain protein (left) and is important in binding the OM to the underlying peptidoglycan, since the C-terminal globular domain contains a peptidoglycan-binding motif (165, 342). A minor fraction of the population, however, folds differently to produce an open β-barrel (right). In E. coli, which produces trimeric, high-permeability porins, the presence of this fraction has no functional consequence. However, in fluorescent pseudomonads, which lack the high-permeability porin, this fraction functions as the major nonspecific porin. This fraction also tends to form a loosely associated oligomeric structure, as shown. The oligomer is shown as a trimer only for illustrative purposes. Modified from reference 455a with permission of the publisher.