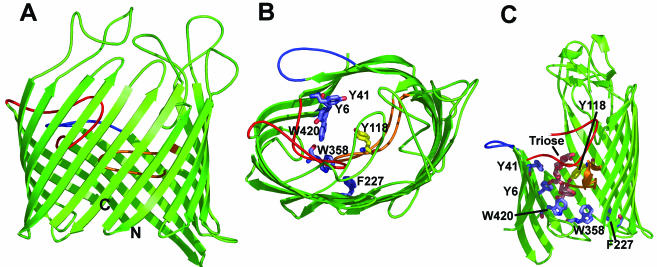
FIG. 4.
X-ray crystallographic structure of LamB. (A) Side view of the monomeric unit. The β-barrel contains 18 strands in this protein, in contrast to the 16 strands seen in the trimeric porins. In addition to loop 3 (orange), loop 1 (red) folds deeply into the channel. Loop 2 (blue) folds outward and interacts with the neighboring subunit in the trimer, as in OmpF (Fig. 2A). Other loops also are often large and tend to cover the entrance of the channel from the outside. (B) View of the monomeric unit from the top. The greasy slides (Tyr41, Tyr6, Trp420, Trp358, and Phe227) are shown as blue stick diagrams, and Tyr118, which constricts the diffusion channel from the opposite side, is shown as a yellow stick diagram. (C) View of the greasy slide and its interaction with maltotriose. This is a side view with the front of the β-barrel cut out for a better view of the slide. The aromatic residues that comprise the greasy slide and Tyr118 are shown as stick diagrams colored as in panel B. The maltotriose molecule (Triose) is shown as a stick diagram colored orange. The coloring of the loops is the same as in panel A. The diagrams are based on PDB coordinate files 1MAL and 1MPN.