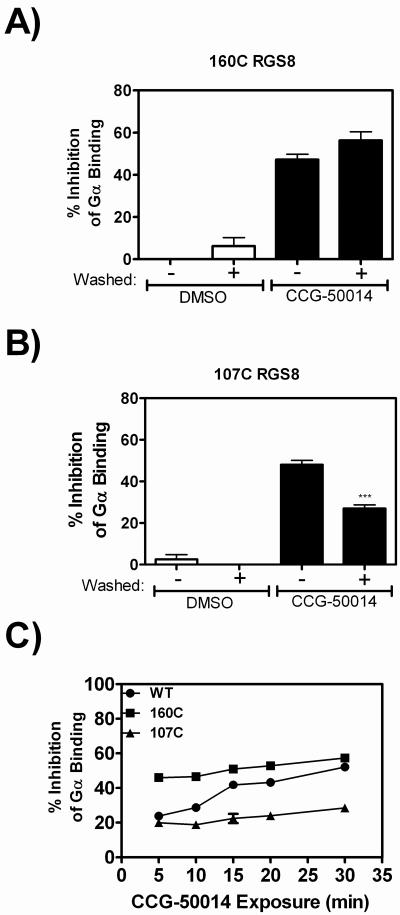
Figure 7. Irreversible inhibition of RGS8 is predominantly mediated by Cys160.
Mutant proteins A) 107C RGS8 and B) 160C RGS8 were pre-bound to beads and exposed to 20 μM CCG-50014, after which reversibility experiments were performed. C) Development of irreversible inhibition after exposure to CCG-50014 differs between the individual cysteine mutants and provides a means to understand the compound's mechanism of action. Wild-type, 160C or 107C RGS8 was treated with 20 μM CCG-50014 for the indicated amount of time before compound removal by extensive washing. The amount of irreversible inhibition was quantified by comparing the G-protein binding to CCG-50014 treated beads to DMSO treated beads. The total amount of inhibition (without a wash step) at this concentration of CCG-50014 for each protein was as follows: WT RGS8: 64±2%; 107C RGS8: 45±2%; and 160C RGS8: 56±1%. Data are presented as the mean±SEM from three independent experiments. ***P<0.0001 using an unpaired t test.